Investigations on the Beneficial Effects of BICOM Optima® Mobile Bioresonance Device on Cultured Connective Tissue Fibroblasts
Dartsch PC*
Dartsch Scientific GmbH, Institut für zellbiologische Testsysteme, Auf der Voßhardt 25, D-49419 Wagenfeld, Germany
Abstract
Background
The holistic bioresonance method uses the electromagnetic waves it receives from the patient and alters the energy field of the organism. Thus, it can be used as a diagnostic and therapeutic method to improve well-being and the success in the treatment of various diseases.
Experimental Principle
In this preclinical and experimental study we used connective tissue fibroblasts to investigate whether the program chain “pathogene Ai” of the BICOM optima® mobile bioresonance device has a positive impact on vitality and regeneration/wound healing process of these cells. Since both cell properties are directly related to each other, an established cell culture test system simulating the granulation stage of wound healing, which is characterized by an increased cell migration and proliferation, was used.
Results
The results show that treatment of cells with the BICOM optima® mobile bioresonance device resulted in a significant improvement of cell vitality by 38.0 ±14.5 % (mean value ± standard deviation; p ≤ 0.01; two-tailed Wilcoxon-Mann-Whitney test) when compared with untreated controls. Moreover, in the cell regeneration/wound healing test system, a cell migration distance of 300 ± 34 μm (mean value ± standard deviation) was measured for bioresonance-treated cells. For untreated cells a migration distance of only 166 ± 38 μm (mean value ± standard deviation) was examined. When calculating the relative values in direct relation, bioresonance treated cells improved wound closure by 81.1 ± 9.2 % (mean value ± standard deviation; p ≤ 0.01; two-tailed Wilcoxon-Mann-Whitney test) when compared with untreated cells.
Conclusions
In summary, the BICOM optima® mobile bioresonance device has demonstrated its beneficial potential on the experimental cellular level. The results confirm previous findings of others on the human body after bioresonance intervention.
Keywords: Bioresonance; Cell vitality; Cell regeneration; Wound healing; L-929; Connective tissue fibroblast; Cell culture
Introduction
Although conventional medicine has made lots of new diagnostic and therapeutic approaches in the last 100 years, a need of a complementary and alternative medicine in order to maintain and improve our well-being arises as a more individualized and holistic point of view.
It is well known that the cells of our body emit and receive electromagnetic signals which allow an intra- and intercellular electromagnetic communication [1,2]. In case of disorders or diseases, this kind of cellular communication is disturbed [3]. The holistic bioresonance method uses the electromagnetic waves it receives from the patient and alters the energy field of the organism. Thus, it can be used as a diagnostic and therapeutic method to improve well-being and the success in the treatment of various diseases [4-6]. For example, Karakos et al. [4] have conducted a recent clinical study with more than 300 patients with nasal, eye, respiratory, cutaneous and gastrointestinal symptoms. After bioresonance treatment, 90 % of the patients observed no more symptoms at all or showed a significant improvement of their symptoms after a bioresonance treatment period of 12 months.
Since preclinical and experimental studies with mammalian cell cultures on the effect of bioresonance are quite rare [7], we conducted this present investigation on cell vitality and regeneration/wound healing of normal connective tissue cells. Since both cell properties are directly related to each other, an established cell culture model simulating the granulation stage of wound healing [8-11] was used to examine whether the BICOM optima® mobile bioresonance device has a positive impact on this complex process.
Materials and Methods
BICOM® Bioresonance Device
A BICOM optima® mobile bioresonance device (Figure. 1) equipped with the BICOM® power applicator GST71 (Figure. 2) was kindly provided by REGUMED Regulative Medizintechnik GmbH, D-82152 Planegg, Germany, for the duration of the experiments for several months.
The program chain “pathogene Ai” was used as the basis. All three single programs of the program chain were set to a duration of 30 min, so that a complete treatment cycle was 90 min. This cycle ran twice in succession when the cell cultures were treated. Thus, the cells were treated for a total of 180 min. The input cup at the top right (channel no. 1; see Figure. 1) was always filled with the same culture medium for the experiments as the cell samples. Of course, the duration of an intervention in humans should not exceed one hour. However, the primary question of this study was to check whether the BICOM® resonance device could actually achieve a reaction of the cultivated connective tissue fibroblasts.

Figure 1: Presentation of the BICOM optima® mobile bioresonance device used with the program chain “pathogene Ai” for the experiments. Note that cell exposure was extended to 180 min and the information in the display that the therapy duration of one hour should not be exceeded. The device was connected with a BICOM® power applicator GST71 as depicted in Figure 2. Also note the red culture medium filled in the input cup at the top right position (channel no. 1).

Figure 2: Arrangement of the power applicator within the external mini-incubator and a cell culture dish in direct contact with the applicator during treatment with the BICOM optima® mobile bioresonance device (Figure. 1).
Routine Cell culture
The investigations were conducted with connective tissue fibroblasts of the cell line L-929 (ACC-2; Leibniz Institute; DSMZ – German Collection for Microorganisms and Cell Cultures, Braunschweig) and used in the subcultivation stages (passages) 40 to 57 over a period of several months. Cells were routinely cultivated in RPMI 1640 with 10 % growth mixture and 0.5 % gentamycin and kept in an incubator at 37 °C with an atmosphere of 5 % CO2 and 95 % air and a humidity of at least 98 %. All cell culture reagent were from PAN-Biotech, Aidenbach, Germany.
Examination of Cell Vitality
For each experiment, the cells from mass cultures were seeded at a cell density of 20,000 cells/well in 24 central wells of two 96- well culture plates (200 μl culture medium/well) and incubated for 24 h until the cells were completely attached and spread. As reference cells for the input cup at the top right of the BICOM® bioresonance device, the cells were seeded in the same density on round glass cover slips with a diameter of 16 mm and preincubated for 24 h.
The previous culture medium was then replaced by 200 μl of Leibowitz L-15 medium with 10 % growth mixture and 0.5 % gentamycin. This special medium ensured a constant pH value of 7.4 at normal atmosphere with its low CO2 content.
The first culture plate was placed directly on the power applicator in a temperature-controlled external mini incubator (Cultura M; Almedica, Switzerland) and treated for a total of 180 min to the program chain “pathogene Ai”. As a reference, the cells on the round glass coverslip were placed into the input cup (channel 1) of the BICOM® device. The second culture plate was incubated as the control in another mini-incubator, about 3 m away from the first one. Finally, both culture plates were incubated for another 22 h in the same external incubator until cell vitality was examined.
Finally, culture medium was replaced by a reaction solution consisting of 180 μl of fresh culture medium and 20 μl of XTT (Xenometrix, Allschwil, Switzerland) which was added to each well. The optical density (= color change) was measured after 0 min and 120 min at 37 °C as a differential measurement at delta OD = 450 – 690 nm with an Elisa reader (BioTEK Elx 808 with software Gen 5/3.00) and compared with each other. XTT is the sodium salt of 2,3-bis [2-methoxy-4-nitro-5-sulfopheny]-2H-tetrazolium-5-carboxyanilide and has a yellowish color. Mitochondrial dehydrogenases of living cells cleave the tetrazolium ring of XTT and orange-colored, water-soluble formazan crystals are formed. The intensity of the orange color of the reaction solution is directly related to cell vitality/metabolic activity [12-14].
Examination of Cell Regeneration/Wound Healing
As previously described in detail elsewhere [15-17], connective tissue fibroblasts were seeded at a density of 50,000 cells/ml into the three individual compartments of a silicone 3 well-culture insert which was attached to the glass bottom of a μ-dish (both from ibidi, Gräfelfing, Germany). The single compartments of the inserts are separated by a 500 μm thick silicone bar with an outer silicone frame of 700 μm. Due to the special adhesion area, an insert adheres firmly to the bottom of a μ-dish and forms a distinct cell-free space (artificial wound), which the cells can close by migration and proliferation.
Upon reaching confluency within 48 h after cell seeding, the silicone inserts were carefully removed with tweezers to achieve a sharp edge of the cell-free space between the compartments. The standard culture medium was replaced by Leibowitz L-15 medium to keep the pH at 7.4 at normal atmospheric conditions. Treatment of cell cultures to the program chain “pathogene Ai” of the BICOM® bioresonance device for 180 min was done as described. Control cell cultures were incubated in the second incubator under the same conditions.
After the treatment period was finished, L-15 medium was replaced by the standard culture medium and the cell cultures were incubated at standard conditions for another 21 h to allow the cells to migrate and proliferate into the cell-free space. Then, cells were fixed with 100 % methanol, stained with Giemsa’s azur eosin methylene blue solution (Merck, Darmstadt, Germany) and were air-dried. The width of the remaining cell-free space was measured by micrographical methods at 4 different cell layer edges with triplicate measurements at each edge for each cell culture. Therefore, for each experimental situation 2 x 24 data points were taken in a single experiment for treated culture and untreated control. The resulting mean values with and without BICOM® bioresonance treatment were used for the final evaluation.
Statistical Analysis
Statistical analysis was done by using the non-parametric two-tailed Wilcoxon-Mann-Whitney test.
Results
As shown in Figure 3, treatment of the cells with the program chain “pathogene Ai” for 180 min resulted in a pronounced improvement of cell vitality when compared with untreated control cells. The difference between BICOM® treated cells and untreated control cells became even more obvious when the relative stimulation was calculated (Figure 4). The stimulation of cell vitality by use of the BICOM optima® mobile bioresonance device in comparison to untreated control cells was 38.0 ± 14.5 % (mean value ± standard deviation). According to the Wilcoxon-Mann-Whitney test the stimulation was statistically significant (p ≤ 0.01). According to the stimulation of cell vitality, treatment of the cultivated connective tissue cells with the program chain “pathogene Ai” of the BICOM optima® mobile bioresonance device also caused the cells to migrate and proliferate much faster. Consequently, the closure of the cell-free space was more apparent within 21 h when compared with the untreated control (Figure 5).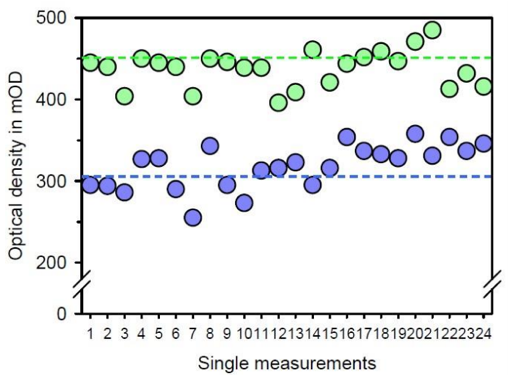

Figure 3: Presentation of the absolute measuring values for cell vitality after treatment with the BICOM optima® mobile bioresonance device and the program chain “pathogene Ai” for 180 min (green data points) in comparison to the untreated control (blue data points). The individual pairs of measured values from the same experiment in the two 96-well culture plates are plotted and the mean value for each experimental situation is given as a dashed line in the appropriate color. It can be easily seen that, despite the variations of a biological system, the mean value for the treated cells is markedly higher than the one for the untreated cells.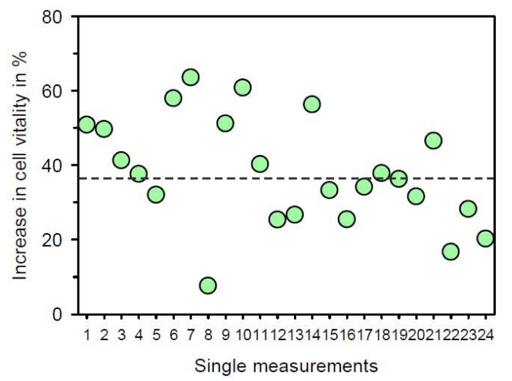

Figure 4: Presentation of the relative increase in cell vitality after treatment with the BICOM optima® mobile bioresonance device and the program chain “pathogene Ai” for 180 min in comparison to untreated control cell culture. The dashed line represents the mean value of the single measurements. The absolute value of the control is always set as “0 %”.
When the wound closure was evaluated by the migration distance of the connective tissue fibroblasts within 21 h after treatment with the bioresonance device, a cell migration distance of 300 ± 34 μm (mean value ± standard deviation) was measured. For the untreated control cells a value of 166 ± 38 μm (mean value ± standard deviation) was examined. Thus, when calculating the relative values in direct relation, the BICOM optima® mobile bioresonance device improved wound closure by 81.1 ± 9.2 % (mean value ± standard deviation; p ≤ 0.01). In additional follow-up experiments conducted with both, μ-dishes with glass and plastic bottom, similar results were obtained.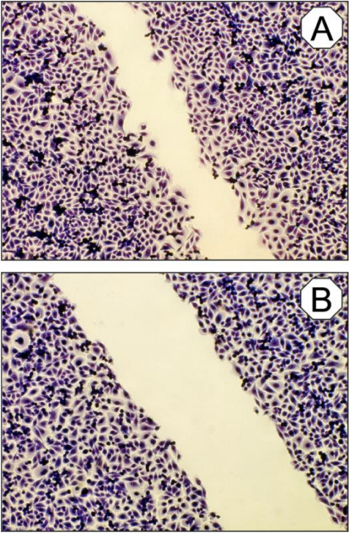

Figure 5: Micrographs of cultured connective tissue cells after an incubation period of 21 h and after treatment with the BICOM optima® mobile bioresonance device and the program chain “pathogene Ai” for 180 min (A) in comparison to the un- treated control culture (B). Olympus IX 50 inverted microscope with planachromate 10x and Olympus E-10 digital camera at 4 megapixel resolution at bright field illumination.
Discussion and Conclusion
Although the bioresonance principles are not really accepted in conventional medicine as a method for diagnosis and therapy, the present investigation has shown that bioresonance treatment obviously has an effect on the cellular level. One might argue that cell cultures are not similar to the complexicity of the human body, but it should be considered that cell cultures can focus on single aspects of living matter as done here. The investigation has two main topics, namely cell vitality which is represented by the metabolic activity of a cell population including mitotic activity (= proliferation) and migration. Based on this consideration, it makes sense to have a closer look on the cell regeneration/wound healing process, because this feature of the cells is the result of both processes, migration and proliferation. However, the terms regeneration and wound healing are often used synonymously and describe one of the most complex biological processes that occur during human life [for review, see 18]. Complicated wound healing processes might also be associated with the occurrence of an excess of oxygen radicals which cause a local oxidative stress in the tissue [19,20]. Prompted by this background we have also investigated the effect of the bioresonance device on the formation of reactive oxygen species by phagocytes and have found that the same program chain as used here is able to inactivate a remarkable part of the superoxide anion radicals formed in the course of an inflammatory process (not shown here).
In the investigation presented here, the BICOM optima® mobile bioresonance device has demonstrated its potential to increase not only vitality of cultivated connective tissue fibroblasts, but also the regeneration/wound healing process. This is a new preclinical finding and an indication that the bioresonance intervention has its beneficial health effects not only in case studies [4], but also on the experimental cellular level. Although bioresonance principles and interventions are not really accepted in wound healing management in conventional medicine, the intervention might be useful in cases with complicated lesions and wound healing processes as a complement to conventional therapies.
In conclusion, the BICOM optima® mobile bioresonance device has demonstrated its beneficial potential on the experimental cellular level. By use of cultivated connective tissue fibroblasts, the present preclinical investigation has shown that a significantly improved cell vitality and regeneration/wound healing process in vitro was caused by the bioresonance treatment due to an increased fibroblast migration and proliferation. The results confirm findings on the human body after bioresonance intervention.
References
1. Prasad A, Rossi C, Lamponi S, Pospíšil P, Foletti A. New perspective in cell communication: Potential role of ultra-weak photon emission. J Photochem Photobiol B. 2014; 139: 47-53.
2. Vladimirsky EB, Milman VD. Mechanisms of signal transduction in cells facts and hypotheses. J Clin Med Sci. 2019; 3: 112.
3. Alberto F, Mario L, Sara P, Settimio G, Antonella L. Electromagnetic information delivery as a new tool in translational medicine. Int J Clin Exp Med. 2014; 7: 2550-2556.
4. Karakos P, Grigorios T, Theodoros K, Theodoros L. The effectiveness of bioresonance method on human health. Open Epidemiol J. 2019; 8: 1-8.
5. Hennecke J. Bioresonance: A New View of Medicin. Scientific principles and practical experience. Books on Demand, Norderstedt. 2012.
6. Ebrahimi M, Sharifov S, Salili M, Chernosova L. An Introduction to Impact of Bio-Resonance Technology in Genetics and
Epigenetics. Epigenetics Territory and Cancer. 2015: 495-513.
7. Podchernyaeva RJ, Lopatina OA, Mikhailova GR, Baklanova OV, Danlibaeva GA, Gushina EA. Effect of exogenous frequency exposure on human cells. Bull Exp Biol Med. 2008; 146: 148-152.
8. Witte M, Barbul A. General principles of wound healing. Surg Clin North Am. 1997; 77: 509-528.
9. Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999; 341: 738-746.
10. Broughton II G, Janis JE, Attinger CE. The basic science of wound healing. Plastic Reconstruct Surg. 2006; 117: 12-34.
11. Wallace HA, Basehore BM, Zito PM. Wound Healing Phases. In: StatPearls. StatPearls Publishing, Treasure Island (FL). 2017.
12. Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Meth. 1991; 142: 257-265.
13. Berridge M, Tan A, McCoy K, Wang R. The biochemical and cellular basis of cell proliferation assays that use tetrazolium salts. Biochemica. 1996; 4:1 4-19.
14. Uzunoglu S, Karaca B, Atmaca H, Kisim A, Sezgin C, Karabulut B, Uslu R. Comparison of XTT and Alamar blue assays in the assessment of the viability of various human cancer cell lines by AT-101 (−/− gossypol). Toxicol Mech Meth. 2010; 20: 482-486.
15. Dartsch PC. Investigations on the beneficial cellular effects of tap water before and after treatment with Alpha Omega (AO) water activator. Jpn J Med. 2018; 2: 330-334.
16. Dartsch PC. Effects of a biophoton triggering device after vitalisation of organ-specific cell cultures. Jpn J Med. 2020; 3: 408-411.
17. Dartsch PC. Beneficial effects of Powerinsole® energy pad: Investigations with organ-specific cell cultures. J Med Stud Res. 2020; 3: 016.
18. Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008; 453: 314-332.
19. Cano Sanchez M, Lancel, S, Boulanger, E, Neviere R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: A systematic review. Antioxidants. 2018; 7: 98.
20. Schafer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res. 2008; 58: 165-171.

