Effects of the BICOM® Optima Mobile Bioresonance Device on Cell Metabolism and Oxidative Burst of Inflammation-Mediating Cells
Peter C Dartsch
Dartsch Scientific GmbH, Institut für zellbiologische Testsysteme, Germany
ABSTRACT
Background: Neutrophils are the most common type of granulocyte, a type of white blood cell, found in most mammals. They play an essential part of the innate immune system and as inflammation-mediating cells. The holistic bio resonance method uses the electromagnetic waves it receives from the patient and alters the energy field of the organism. Thus, it can be used as a diagnostic and therapeutic method. Prompted by this background we investigated whether the treatment with the BICOM® optima mobile bioresonance device could achieve an anti-inflammatory response of cultivated functional neutrophils by reducing the metabolic activity and the generation of superoxide anion radicals which might cause local oxidative stress in the inflamed tissue.
Material and Methods: Human promyelocytes (HL-60 cells) were differentiated to functional neutrophils over a period of 5 days by the addition of 1.5 vol% dimethyl sulfoxide to the culture medium. During the differentiation process, the cells were treated with the program chain “pathogene Ai” of the BICOM® optima mobile bioresonance device on three consecutive days in an external mini-incubator at 37 °C for 180 min each. The controls were incubated for the same time in another external mini-incubator without bio resonance treatment. Finally, the basal cell metabolism and the generation of superoxide anion radicals with and without treatment of the functional neutrophils was examined.
Results: Treatment with the BICOM® mobile bioresonance device had no significant influence on mitotic activity or cell size of functional neutrophils. Measurement of the basal cell metabolism of the functional neutrophils without triggering an oxidative burst resulted in a statistically significant reduction of the basal cell metabolism by 12.2 ± 4.5 % (mean value ± standard deviation; p ≤ 0.05; Wilcoxon-Mann-Whitney test). In accordance with the reduction of the basal metabolism was the reduction in the generation of superoxide anion radicals after treatment with the BICOM® mobile bioresonance device by 17.8 ± 3.1% (mean value ± standard deviation). When compared with the untreated control, this inhibition of radical formation was also statistically significant (p ≤ 0.01; Wilcoxon-Mann-Whitney test).
Conclusion: Bioresonance principles are not really accepted in the treatment of acute and chronic inflammatory processes or in wound healing management in conventional medicine. However, the results of this investigation suggest that bioresonance intervention might be very useful in the treatment of acute and chronic inflammatory processes as a complement to conventional therapeutic approaches. Moreover, a treatment with the BICOM® optima mobile bioresonance device might improve and maintain health and well-being.
Keywords: Bio Resonance; Inflammation; Oxidative Burst; Neutrophil; HL-60; Cell Culture
Introduction
Neutrophils are the most common type of granulocyte, a type of white blood cell, found in most mammals. They play a two fold role as (i) phagocytes (= scavenger cells) by floating in the circulating blood and forming a cellular defense against invading microbial pathogens as an essential part of the innate immune system [1], and (ii) as inflammation-mediating cells after having entered the inflamed tissue. In the case of inflammation, the neutrophils migrate from the blood into the tissue and generate reactive oxygen species, preferably superoxide anion radicals, in a so-called oxidative or respiratory burst [2,3]. Although radicals play an important role in intercellular signal transmission [4,5], an excess of radicals in the tissue causes a local oxidative stress and can no longer be neutralized by the body’s own enzymes such as superoxide dismutase and others [6-8]. The resulting oxidative stress in the tissue causes modifications in biomolecules such as proteins, lipids and DNA. The resulting cell and tissue damage is an initial step in the pathogenesis of multiple diseases [9-12]. It is well known that the cells of our body emit and receive electromagnetic signals which allow an intra- and intercellular electromagnetic communication [13,14]. In case of disorders or diseases, this kind of cellular communication is disturbed [15]. The holistic bioresonance method uses the electromagnetic waves it receives from the patient and alters the energy field of the organism.
Thus, it can be used as a diagnostic and therapeutic method to improve well-being and the success in the treatment of various diseases [16-18]. In a previous study we have already demonstrated that the BICOM® optima mobile bioresonance device is able to promote the wound healing process by a stimulation of the metabolic activity and of cell migration and proliferation of cultured connective tissue fibroblasts [19]. Prompted by this background we investigated in the present study whether the treatment with the BICOM® optima mobile bioresonance device could also achieve an anti-inflammatory response of functional neutrophils by reducing metabolic activity and generation of superoxide anion radicals upon activation.
Material and Methods
BICOM® Bioresonance Device
The BICOM® optima mobile bioresonance device equipped with the BICOM® power applicator GST71 was kindly provided by REGUMED Regulative Medizintechnik GmbH, D-82152 Planegg, Germany, for the duration of the experiments. The program chain “pathogene Ai” was used as the basis. All three single programs of the program chain were set to a duration of 30 minutes, so that a complete treatment cycle was 90 minutes. This cycle was run twice in succession for treatment of the cell cultures. Thus, the cells were treated for a total of 180 minutes. The input cup at the top right was always filled with the same culture medium for the experiments as the cell samples. Of course, the duration of an intervention in humans or animals should not exceed one hour. However, the primary question of this study was to check whether the BICOM® bioresonance device could actually achieve an anti-inflammatory response of functional neutrophils by reducing the metabolic activity and the generation of superoxide anion radicals.
Routine Cell Culture
The investigations were conducted with human promyelocytes (cell line HL-60; ACC-3; ECACC 98070106; Leibniz-Institut; DSMZ German Collection for Microorganisms and Cell Cultures, Braunschweig, Germany) in the sub cultivation stages (passages) 10 to 18 over a period of two months. The cells were routinely cultivated in RPMI 1640 medium supplemented with 10 % growth mixture and 0.5 % gentamycin. Cell cultures were routinely incubated in an incubator at 37 °C and an atmosphere of 5 % CO2 and 95 % air at almost 100 % humidity. At culture conditions with the culture medium containing 1.5 vol% dimethyl sulfoxide, the cells can be differentiated into so-called functional neutrophils which are able to generate superoxide anion radicals by an oxidative burst upon activation by a phorbol ester [20-23].
Experimental Design
Cells were cultivated as suspension cultures in special culture flasks with a ventilated and lockable lid (25 cm2 growth area; TPP, Switzerland) which allows to inhibit gas exchange between the culture medium and the normal air during external treatment so that the pH value remained constant. By the addition of 1.5 vol% dimethyl sulfoxide for a total of 5 days, the HL-60 cells were differentiated into functional neutrophils. During the differentiation process, the cells were treated with the BICOM® optima mobile bioresonance device on three consecutive days in an external mini incubator at 37 °C for 180 min each. The program chain “pathogene Ai” was used as described above. The respective controls were incubated for the same time in another external mini-incubator without bioresonance treatment. The cells were then cultured in the gassed main incubator with open lid until the next exposure. Onday 5 of differentiation, the cells were prepared by centrifugation (6 min at 190 x g) and repeated washings in phosphate buffered saline with calcium and magnesium and 60 μl aliquots of the resuspended cells in the buffer containing 10 mM glucose were taken for thetests. The functional neutrophils in the reaction mixture were activated to generate superoxide anion radicals by adding phorbol-12-myristate-13-acetate (Sigma-Aldrich, Deisenhofen, Germany) [24].
The generation of superoxide anion radicals in the reaction mixture by the cells caused the cleavage of the tetrazolium dye WST-1 (Roche Diagnostics, Mannheim, Germany), which was also present in the reaction mixture. The amount of superoxide anion radicals present in the reaction mixture was directly related to the color change of the dye. In addition, the basal metabolic activity of the functional neutrophils was examined in the same way without activation of the cells to produce an oxidative burst. The optical density was recorded as a differential measurement ∆OD = 450 – 690 nm by an Elisa reader (BioTek SLx 808 with software Gen 5 version 3.00) and evaluated after linear regression for the time interval 0 to 30 min with Microsoft Excel. Five independent tests were conducted. Additionally, by using a CASY cell analysis system (Omni Life Science, Bremen, Germany), three of the tests were also used for analysis of cell number and cell size distribution of the differentiated cells.
Statistical Analysis
Statistical analysis was performed by using the non-parametric two-tailed Wilcoxon-Mann-Whitney test.
Results and Discussion
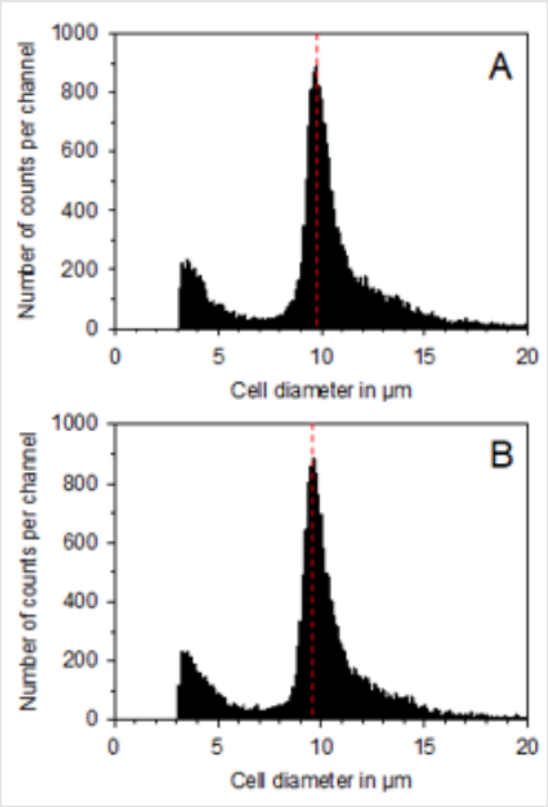

Figure 1: Presentation of the cell size distribution of functional neutrophils without treatment. (A) and after treatment on 3 consecutive days with the BICOM® optima mobile bioresonance device during the 5-day differentiation period from promyelocytes to functional neutrophils. (B) The small peaks at about 3 μm in each distribution represent small particles (so-called cell debris such as cell fragments and dead cells) and are not included in the evaluation.
As shown in (Figure 2), the measurement of the maximum cell diameter resulted in a value of 9.95 ± 0.17 μm for the untreated control cells and a value of 9.79 ± 0.25 μm for the BICOM®-treated cells (mean value ± standard deviation; n = 3). In the BICOM®-treated cells, the cell number was 7.7 ± 4.6 % lower than in the control cells (mean value ± standard deviation; n = 3; not depicted). However, the values for cell diameter and cell numbers were not significantly different between treated and control cells demonstrating that treatment with the BICOM® mobile bioresonance device had no significant influence on proliferative activity or cell size of functional neutrophils. Measurement of the basal cell metabolism of the functional neutrophils without triggering an oxidative burst resulted in a reduction after treatment with the BICOM® optima mobile bioresonance device when compared with untreated controls (Figure 2). When calculating the mean value ± standard deviation from 5 independent experiments, a statistically significant reduction of the basal cell metabolism by 12.2 ± 4.5 % (p ≤ 0.05) was obtained. In accordance with the reduction of the basal metabolism of the functional neutrophils was the reduction in the generation of superoxide anion radicals after treatment with the BICOM® mobile bio resonance device (Figure 2). The reduction in all 5 independent experiments was 17.8 ± 3.1 % (mean value ± standard deviation). When compared with the untreated control, this inhibition of radical formation was also statistically significant (p ≤ 0.01).
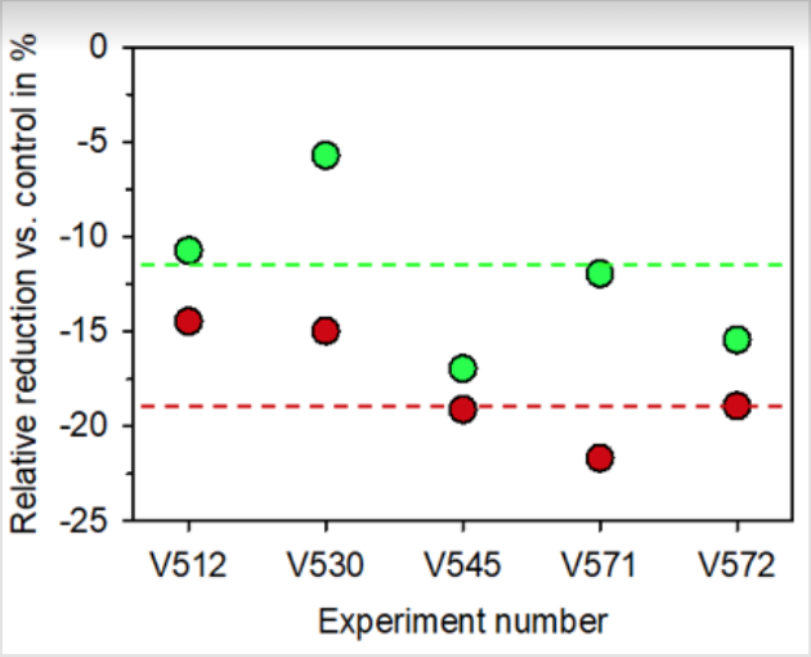

Figure 2: Reduction of the basal metabolic activity (green data points) and radical generation (red data points) of functional neutrophils after daily treatment for three times with the BICOM® optima mobile bioresonance device during the 5-day differentiation process. The results of the 5 independent experiments (V512, V530, V545, V571 and V572) are shown as well as the mean of all experiments in the form of the dashed line (green for metabolic activity and red for superoxide anion radical generation). The untreated controls are set as “0” in each experiment.
Although the bioresonance principles are not really accepted in conventional medicine as a method for diagnosis and therapy, the present investigation has shown that bioresonance treatment obviously has a definite effect on the cellular level. One might argue that cell cultures are not similar to the complexity of the human body, but it should be considered that cell cultures allow to focus on selected aspects of living matter. The investigation presented here uses functional neutrophils ± treatment with the BICOM® optima mobile bioresonance device and has its focus on two principle characteristics of these cells: (i) the metabolic activity and (ii) the generation of superoxide anion radicals in the course of an induced oxidative burst. In a previous investigation we found that treatment of connective tissue fibroblasts with the BICOM® optima mobile bioresonance device resulted in a stimulation of basal cell metabolism and consequently in an improved wound healing [19]. At first sight, the finding of this present study, namely the reduction in metabolic activity and generation of superoxide anion radicals of functional neutrophils, might be somehow contradictory. However, chronical inflammatory and complicated wound healing processes are also associated with the occurrence of an excess of oxygen radicals which cause a local oxidative stress in the tissue [25-27].
From this point of view, the findings from connective tissue fibroblasts and functional neutrophils complement each other by possibly shortening an inflammatory event or processes which are related to wound healing and/or oxidative stress in vivo. A much stronger effect of the BICOM® optima mobile bioresonance device on functional neutrophils would reduce the efficiency of the innate immune defense of the human body against microbial pathogens circulating in the blood.
Conclusion
Bioresonance principles are not really accepted in the treatment of acute and chronic inflammatory processes or in wound healing management in conventional medicine. The results presented here suggest that bioresonance intervention might be very useful in the treatment of acute and chronic inflammatory processes as a complement to conventional therapeutic approaches. Moreover, a treatment with the BICOM® optima mobile bioresonance device might improve and maintain health and well-being.
References
1. Witko Sarsat V, Rieu P, Descamps Latscha B, Lesavre P, Halbwachs Mecarelli L (2000) Neutrophils: molecules, functions, and pathophysiological aspects. Lab Invest 80: 617-653.
2. Nathan C (2002) Points of control in inflammation. Nature 420: 846-852.
3. Ward PA (1999) The acute inflammatory response and its regulation. Arch Surg 134: 666-669.
4. Vliet van der A, Bast A (1992) Effect of oxidative stress on receptors and signal transmission. Chem Biol Interact 85: 95-116.
5. Lander HM (1997) An essential role for free radicals and derived species in signal transduction. FASEB J 11: 118-124.
6. Mc Cords JM, Fridovich I (1969) Superoxide dismutase – an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244: 6049-6055.
7. Culotta VC (2001) Superoxide dismutase, oxidative stress, and cell metabolism. Curr Top Cell Reg 36: 117-132.
8. Limón Pacheco J, Gonsebatt ME (2009) The role of antioxidants and antioxi-dant-related enzymes in protective responses to environmentally induced oxidative stress. Mut Res Gen Toxicol Environ Mutagen 674: 137-147.
9. Kannan K, Jain SK (2000) Oxidative stress and apoptosis. Pathophysiol 7(3): 153-163.
10. Higuchi Y (2003) Chromosomal DNA fragmentation in apoptosis and necrosis induced by oxidative stress. Biochem Pharmacol 66: 1527-1535.
11. Choi K, Kim J, Kim GW, Choi C (2009) Oxidative stress-induced necrotic cell death via mitochondira-dependent burst of reactive oxygen species. Curr Neu-rovasc Res 6: 213-222.
12. Edae CK, Tofik E (2020) Biomarkers of oxidative stress and its role in athero sclerosis development. Biomed J Sci & Tech Res 32: 2020.
13. Prasad A, Rossi C, Lamponi S, Pospíšil P, Foletti A (2014) New perspective in cell communication: Potential role of ultra-weak photon emission. J Photochem Photobiol B 139: 47-53.
14. Vladimirsky EB, Milman VD (2019) Mechanisms of signal transduction in cells facts and hypotheses. J Clin Med Sci 3: 112.
15. Alberto F, Mario L, Sara P, Settimio G, Antonella L (2014) Electromagnetic information delivery as a new tool in translational medicine. Int J Clin Exp Med 7: 2550-2556.
16. Karakos P, Grigorios T, Theodoros K, Theodoros L (2019) The effectiveness of bioresonance method on human health. Open Epidemiol J 8: 1-8.
17. Hennecke J (2012) Bioresonance: A New View of Medicin. Scientific Principles and Practical Experience. Books on Demand, Norderstedt.
18. Ebrahimi M, Sharifov S, Salili M, Chernosova L (2015) An introduction to impact of bio-resonance technology in genetics and epigenetics. In: Mehdipour P (Eds.). Epigenetics Territory and Cancer. Springer, Dordrecht pp. 495-513.
19. Dartsch PC (2021) Investigations on the beneficial effects of BICOM optima mobile bioresonance device on cultured connective tissue fibroblasts. J Biomed Sci Res 3 (1): 133.
20. Babior BM (1999) NADPH oxidase: An update. Blood 93: 1464-1476.
21. Tan AS, Berridge MV (2000) Superoxide produced by activated neutrophils efficiently reduces the tetrazolium salt WST-1 to produce a soluble formazan: A simple colorimetric assay for measuring respiratory burst activation and for screening anti-inflammatory agents. J Immunol Meth 238: 59-68.
22. Teufelhofer O, Weiss RM, Parzefall W, Schulte Hermann R, Micksche M, et al. (2003) Promyelocytic HL60 cells express NADPH oxidase and are excellent targets in a rapid spectrophotometric microplate assay for extracellular superoxide. Toxicol Sci 76: 376-383.
23. Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82: 47-95.
24. Dartsch PC (2006) TIIOS – a sensitive and cell-based test assay for the screening of biologically active substances for their antioxidant potential. Innov Food Technol 32: 72-75.
25. Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453: 314-32.
26. Cano Sanchez M, Lancel S, Boulanger E, Neviere R (2018) Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: A systematic review. Antioxidants 7(8): 98.
27. Schafer M, Werner S (2008) Oxidative stress in normal and impaired wound repair. Pharmacol Res 58: 165-171.

