Speaker: Hans Brügemann, Head of the Institute, Gräfelfing/Germany
Are there evidence-based studies on the efficacy of the bioresonance method?
INTRODUCTION
The phrase „level of evidence“ is often used nowadays when attempting to prove the efficacy of drugs and medical devices. It describes the level of scientific proof and presence of meaningful data. The nature of this evidence is based on findings but more particularly on the construction of a scientific study, now also known as a „design“.
Various schemes have been proposed for classifying the level of evidence. We are using here the 8-level scheme of evidence according to the American Heart Association.
Classification of the levels of evidence according to the American Heart Association (AHA), modified according to W. F. Dick: Evidence-based emergency medicine (abridged):
- Level 1: Statistically significant, randomised, controlled trials (double-blind studies) or meta-analyses
- Level 2: Statistically insignificant, randomised, controlled trials (double-blind studies) or meta-analyses
- Level 3: Prospective, controlled, but not randomised cohort studies
- Level 4: Historic, not randomised cohort or casecontrol studies
- Level 5: Human case series
- Level 6: Animal or mechanical model studies
- Level 7: Reasonable extrapolations from existing data
- Level 8: Rational conjecture
Health insurance companies, public bodies and courts have however increasingly adopted the extreme view that only level 1 randomised, controlled double-blind studies provide scientific proof of efficacy.
However, this is also a very narrow way of looking at the matter and does not do it any justice. One of the leading lawyers in the pharmaceuticals industry admits as much:
A doctor’s opinion is no longer worth anything. The authorities are increasingly turning to policy recommendations. Medicine has been reduced to natural science. The randomised double-blind study is being used in an increasingly restrictive manner.
As is often the case, the truth lies somewhere in between – or needs to be looked at in a different way.
Human case series (level 5) are certainly not meaningful for medical indications with major spontaneous fluctuations and where success is assessed subjectively. But for an indication with spontaneous fluctuations and less pronounced trends towards spontaneous improvement, this type of study has a high level of evidence, however. In this sense a slight improvement in hay fever during a particular season is not meaningful, whereas the recovery or part-recovery of a large number of patients certainly is.
We commissioned Dr. Volker W. Rahlfs, C. Stat. (RSS), Head of the Institute for Data Analysis & Study Planning from Germany, founded in 1966, to carry out an expert analysis of the studies available on BICOM bioresonance therapy. Dr. Rahlfs has 40 years experience as a biometrician/biostatistician in the area of clinical research and has given expert advice and opinion to 140 pharmaceutical companies and university institutes and led more than 400 scientific studies in Germany and overseas.
Dr. Rahlfs was given studies on the use of BICOM Bioresonance Therapy to assess. In his expert report he uses the aforementioned 8-level scheme of evidence classification.
I would now like to give you a more detailed insight into the studies with their results and classification using this scheme of evidence (i. e. assessing the scientific evidence).
ASSESSMENT SUMMARY
The assessor makes the following concluding remarks about the studies:
„All previous studies and research work indicate that the BICOM procedure does not only show statistically significant (and in the sense of random statistics, demonstrable) effects. These are to be interpreted in a clinical context as demonstrating efficacy. Undesirable side effects, particularly those that are serious, are not found in any study.
The work discussed and assessed here corresponds in principle to the quality standard of university research. Evidence level 1 with controlled double-blind studies is not the norm in that area. This quality standard is currently only required in the area of pharmacological research. The documents presented correspond to the requirements of the clinical assessment of medical products. (cf: R. Prestel, Anforderungen an die klinische Bewertung von „bekannten“ Medizinprodukten aus der Sicht einer benannten Stelle [Clinical assessment requirements of „known“ medical products from the point of view of a Notified Body], Medizintechnik 121 (2001) 9-13.)“
The assessor goes on to sum up his assessment as follows:
“It is standard practice worldwide to publish your own results, even those with a low level of evidence and, as demonstrated in the present report, to derive the level of evidence from the reproducibility. In practical terms this means that even studies with a lower level of evidence are considered as providing proof if other researchers – who are also carrying out studies with a lower level of evidence – come to the same conclusions.
This generally recognised technique of external validation can be seen in the studies appraised here, carried out by Huang S. et al. (2005), Yang J. Zhang (2004) and Zhang X. et al. (2005) in which the named authors each compare their findings with the results of other authors in their publications.“
Summary: The studies carried out using the BICOM method were appraised by experts Dr. Volker W. Rahlfs, C. Stat. (RSS) and Dr. med. Andreas Rozehnal from the idv Institute for Data Analysis & Study Planning as follows.
4 studies were awarded a level of evidence 1
1 study was awarded a level of evidence 1-2
1 study was awarded a level of evidence 2
1 study was awarded a level of evidence 3
4 studies were awarded a level of evidence 4-5
4 studies were awarded a level of evidence 5
All clinical studies were carried out without our knowledge, i. e. the studies were not commissioned, which further increases the evidentiary power of the studies presented.
Is it now possible to claim that the efficacy of BICOM Bioresonance Method is scientifically proven? Yes. Anyone suggesting otherwise is ignoring these studies.
BRIEF PRESENTATION OF THE STUDIES, THEIR RESULTS AND ASSESSMENTS
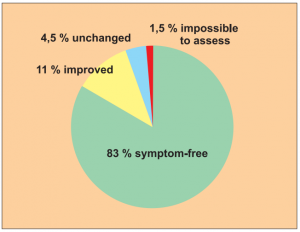 Study 1:
Study 1:
Single group cohort study1 with 204 cases of allergy patients with different strains.
Author: Schumacher, P.
The results of this study should be well known within our circles. Nevertheless I would like to reproduce the results in a pie chart.
The biometric/medical assessment: „For this indication spontaneous healing is extremely rare. There is no known evidence of healing using therapeutic measures. Therefore an 83 % recovery rate is an extremely convincing statistic (15 % recovery rate would be deemed of clinical significance). Level of evidence: 4/5“.
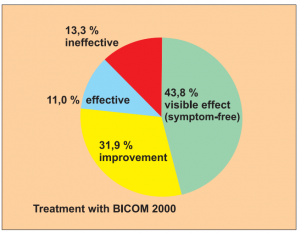
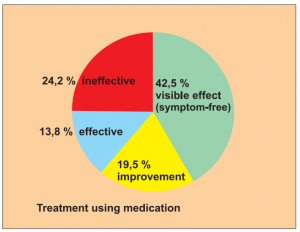
Study 2:
Prospective, controlled but not randomised study with 2 groups: 213 patients treated with BICOM, 87 patients with corticoids and anti-allergy medication. Study of patients with asthma.
Study carried out by: Yang Jinzh and Zhang Li, Research Centre of the Jian Paediatric Clinic for the Prevention and Treatment of Asthma.
The results of the treatment were classified after 6 months as:
- Visible effect (symptom-free)
- Improvement
- Effectiveness (slight reduction)
- Ineffectiveness
 1 Study of a group of patients (not randomised)
1 Study of a group of patients (not randomised)
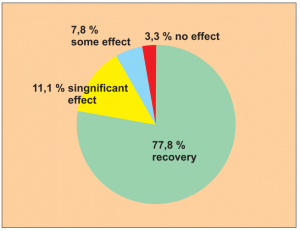
Study 3:
Single group cohort study with serial observation of 154 allergy patients from June 2002 to January 2004. Dermatitis, rhinitis, allergic conjunctivitis and asthma were treated. Immediately before and during treatment no anti-allergy medication was taken.
Study carried out by: Yuan Ze, Huang Jiali, Wang Haiyan and Yu Chunyan, Xian Department of Paediatrics, Central Hospital, Xi’an.
Following treatment 120 out of 154 patients (= 78 %) recovered fully (symptom-free for 6 months). No undesirable effects were reported.
Extract from the assessment: Level of evidence 4/5. This is based on diagnoses which, if using conventional medical treatment, in practical terms may only be controlled to a certain extent with long-term medication (e. g. corticoids) which has a number of side effects.
The results were looked at and analysed 6 months after patients received treatment.
Study 4:
Cohort study with serial observation of 1639 patients with different allergy diagnoses. These are patients who had all been unsuccessfully treated in the past with standard medication.
The study was carried out in the Paediatrics Dept. of the Central Hospital in Xi’an, China. Authors: Ze Y. und Haiyan W.
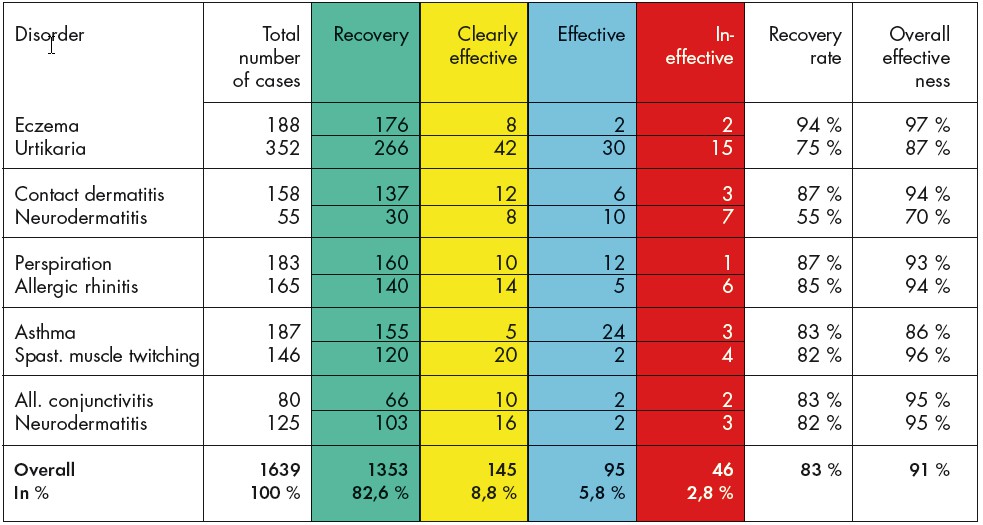 Extract from the assessment: The patients had been treated in the past with various medications with little success. No recurrence of symptoms 6 months after BICOM therapy meant, for this period at least, that patients were cured. Spontaneous healing, placebo effects and similar cannot explain the percentage of patients who made a recovery in this allergy area. Level of evidence 4-5.
Extract from the assessment: The patients had been treated in the past with various medications with little success. No recurrence of symptoms 6 months after BICOM therapy meant, for this period at least, that patients were cured. Spontaneous healing, placebo effects and similar cannot explain the percentage of patients who made a recovery in this allergy area. Level of evidence 4-5.
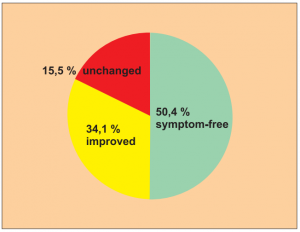
Study 5:
Single group cohort study of 200 patients from a total of 248 questionnaires sent out. Patients with a longer case history (as well as various treatments prior to this): allergically related skin disorders (neurodermatitis, eczema, pruritus), allergic conjunctivitis, allergic intestinal disorders, allergic respiratory disorders, pollen allergies.
Author: Hennecke, J.
Treatments were carried out without allergen abstinence.
Extract from the assessment: Despite possible distortion of the result it can be assumed that a substantial number of
patients were symptom-free (80.6 % return rate from the postal questionnaire).
The number of symptom-free or improved patients can certainly not be explained by placebo effects or misdiagnosis.
Level of evidence 4/5.
Study 6:
Prospective randomised parallel 2-group study with 2 x 14 patients with liver cell damage.
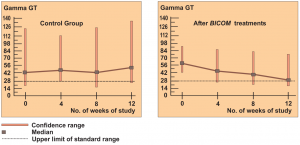
The 2 groups were made up of patients with liver cell damage who had been diagnosed at least one year before. The enzyme values in the control group were almost unchanged around the median value and also remained largely pathological in individual patients (see the diagrams on following page).
In the group treated with BICOM a considerable improvement can be seen in the median. The individual values are normalised in most of the patients. The differences between the groups are both substantially and statistically significant.
Authors: Machowinski, R. und Gerlach, I.
Assessment: The study does not only show significant differences from the control group but the effects are also quite considerable and of medical significance. The design of the study, awarded level of evidence 1, suggests a statistically sound and quite considerable level of efficacy for this indication.
Diagram a) Study 6 – Laboratory results liver enzyme GOT
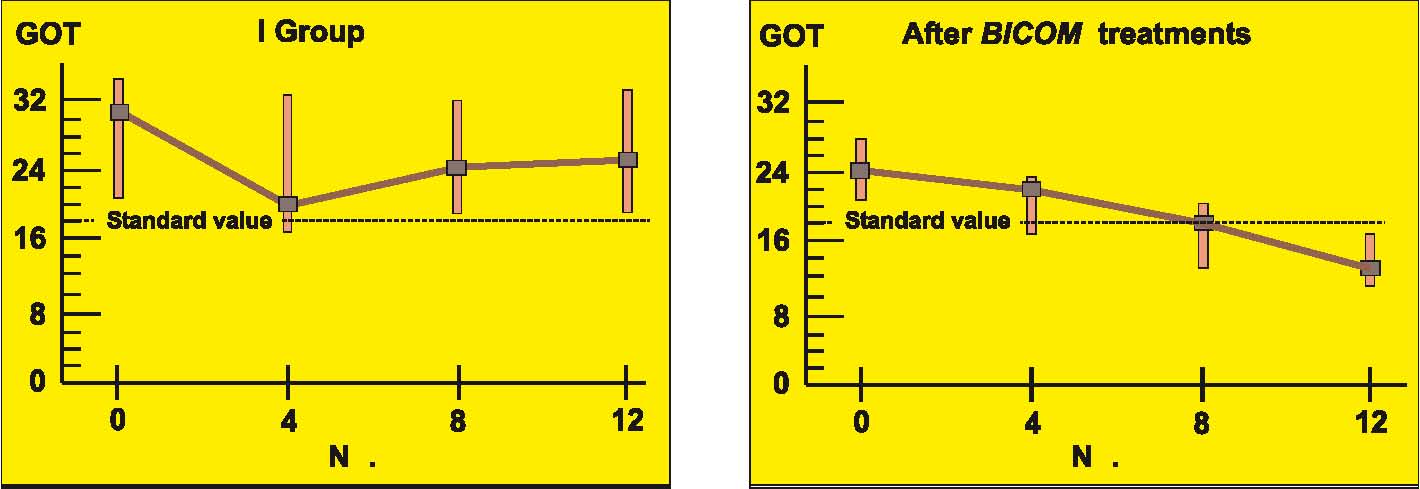
Diagram b) Study 6 – Laboratory results liver enzyme GPT
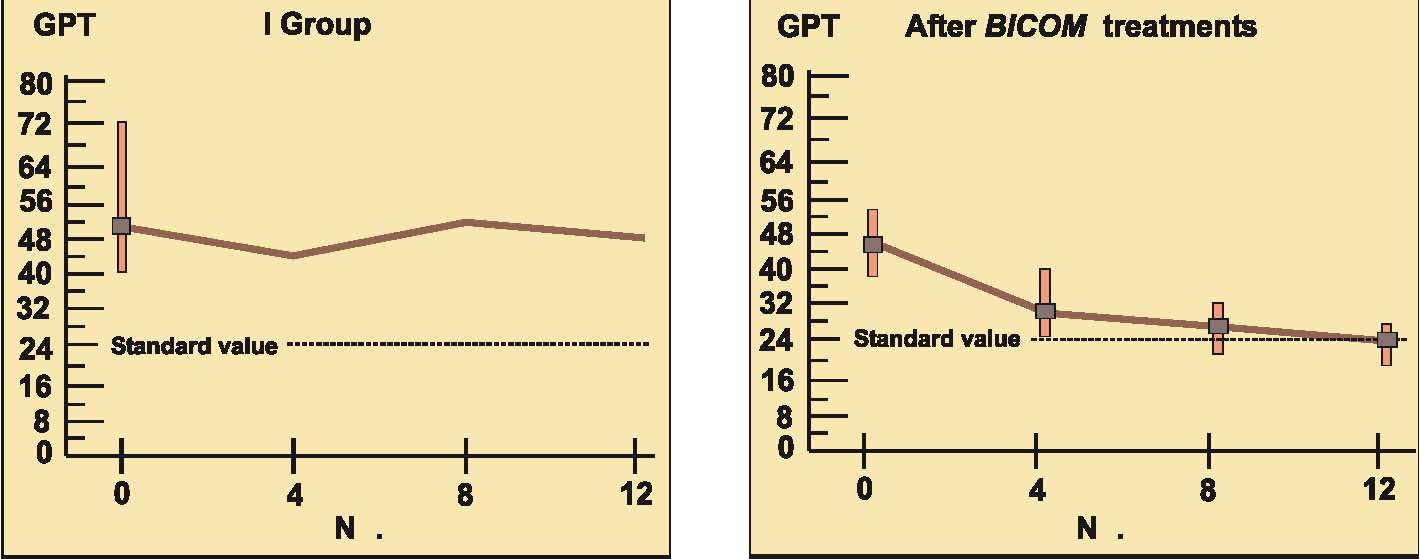
Diagram c) Study 6 – Laboratory results liver enzyme Gamma GT
Study 7:
Two groups of athletes, not randomised, 12 patients in each group, suffering from overstrain syndromes associated with high performance athletes.
Study carried out by: Papez, B. J. and Barovic, Maribor Teaching Hospital, Slovenia, Dept. of Medical Rehabilitation.
The control group was treated with ultrasound as well as cryotherapy and electro-stimulation treatment. The test group only received BICOM Bioresonance Therapy.
Diagram a) Study 7
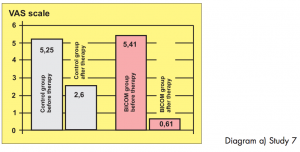
Diagram b) Study 7
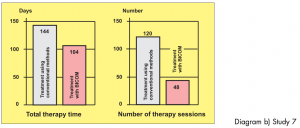
Assessment: „Based on the 8-point level of evidence scale, the study is awarded level 2 in terms of design i. e. providing strong evidence. In this sense the efficacy is shown to be statistically significant. The extent of the efficacy is also considerable both in terms of length of therapy and pain score.“
Study 8:
Controlled pre-clinical in-vitro study.
Summary illustration of in-vitro modulation of the phagocyte activity of human polymorph nuclear leucocytes through BICOM therapy. A total of 50,000 blood samples were treated and checked using various program parameters.
The study was carried out by O. Osadchaya et al. at the Kavetzky Institute for Experimental Pathology, Oncology and
Radiobiology at the Ukraine State Academy of Sciences. Level of evidence 1.
The phagocytic activity of human phagocytes in donor blood was statistically significantly altered through BICOM treatment. In-vitro study – controlled study.
The study shows clearly different and reproducible results using various program parameters. The phagocytic activity
of human phagocytes in donor blood was altered through BICOM treatment to a statistically significant extent.
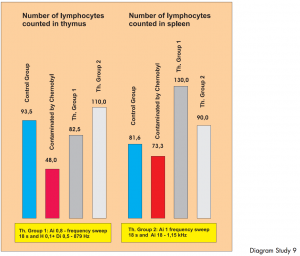
Diagram Study 8
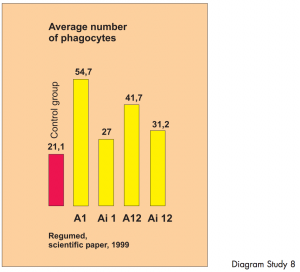
The first bar shows the number of phagocytes in the donor blood. The blood was placed in 10 test tubes in the input cup and 10 ampoules with the same donor blood were also placed in the output cup. „Treatment“ was carried out using different therapy programs. A and Ai denote the type of therapy and the additional figures show the amplifications. The next four bars show the number of activated phagocytes following each BICOM therapy session.
Study 9:
Controlled pre-clinical in-vitro study: investigation into the reproduction of the immune system of radioactively contaminated mice.
Carried out by D. Sakharov et al.
Through BICOM treatment it was possible to return the immune systems of mice weakened by radioactivity in Chernobyl to a statistically significant and relevant normal level. Level of evidence 1.
Diagram Study 9
Study 10:
Comparative diagnostic study: BICOM bioresonance test versus prick test.
31 subjects were each tested with a prick test and BICOM test for mites, grasses, olive, wall pellitory.
The study was carried out by the doctors Giannazo, Valenti and Puzzo from the Physiology Dept., Chair of Biophysics at the University of Catania. 31 double readings were taken on 4 occasions.
The sensitivity of BICOM is 0.84 (95 % CI: 0.72-0.92). The specificity is 0.66 (95 % CI: 0.53-0.78). These two amounts define the „true positive“ and „true negative“ of certain cases.
Youden’s index combines both masses and it is exactly 0.5 (95 % CI: 0.34-0.64. Lower limit 0 = useless test; 1.0 = perfect test).
The biometric assessment: The BICOM device is certainly suitable as an objective procedure for carrying out allergy testing. It would be desirable to carry out further investigations in which the accuracy of both the prick tests and the BICOM tests could be determined using a „gold“ standard and discussed accordingly.
Level of evidence 1.
Study 11:
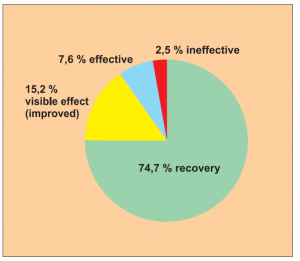
Single group cohort study with clearly defined efficacy criteria.
The study is sufficiently representative with 79 patients taking part. Included in the study are eczema, ongoing dermatitis, nettle rash and psoriasis.
The study was carried out by Dr. Du Xia et al.
The efficacy was assessed using a 4-point scale. The follow-up observation after 1 year is notably long and increases confidence in the results of the study in terms of evidence-based medicine.
Result: Recovery in 74.7 % of treated cases and a visible effect in a total of 89.9 % of cases observed.
Assessment: The study was given a level of evidence 5.
Diagram Study 11
Study 12:
Single group cohort study with clearly defined efficacy criteria. Despite the lack of a comparison group, it appears to be a clear indication of the efficacy (Diagram 12).
The study comprised 150 patients in total, made up as follows: 95 patients with asthma and nasal catarrh, 20 patients with asthma only, 25 patients with nasal catarrh, 5 patients with skin eczema, 5 patients with other allergies.
This study was carried out by Dr. Feng Y. et al.
Extract from the biometric/medical assessment: It seems to be a clear indication of the efficacy despite the lack of a comparison group since the successful results significantly outweigh the anticipated random effect. The credibility of the diagnoses for inclusion is supported by reference to relevant criteria. Level of evidence 5.
Diagram Study 12
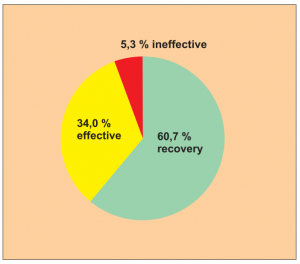
Efficacy was checked using a 3-point scale. In 60.7 % of the cases all symptoms had disappeared. The general efficacy was proven at 94.7 % and a long-term recovery could also be seen in this study.
Treatment comprised 5 to 8 sessions. It was considered to be finished if all allergies tested negative in a renewed check. The observation period covered 5 to 8 sessions.
Study 13:
Prospective randomised controlled parallel group study (Diagram 13). The patients were distributed into 3 groups.
Group 1: BICOM treatment for children with first time diagnosis Group 2: BICOM treatment for children who were previously unsuccessfully treated with medication
Group 3: Control group, children with first-time diagnosis, treatment with medication
181 patients with allergy-related colds and allergic bronchial asthma were included in this study.
Diagram a) Study 13 & Diagram b) Study 13
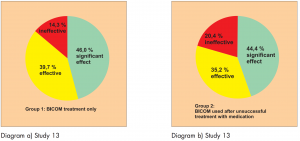
Diagram c) Study 13
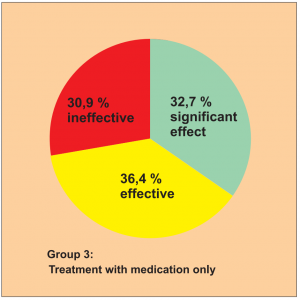
The study was carried out by Dr. Huang S. et al.
The efficacy was assessed using a 3-point scale: significant effect, effective, ineffective. The success rate is shown in the following diagrams.
This study is awarded a level of evidence 1-2 based on the comparison groups available.
Study 14:
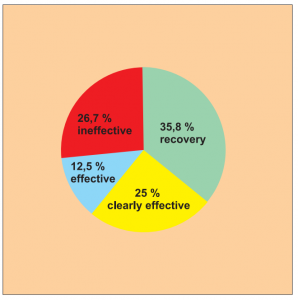
Single group cohort study. 56 patients across all age groups suffering from nettle rash took part in this study.
It was carried out by Dr. Xu M. et al.
The results were assessed on a 4-point scale: recovery, clearly effective, effective (with relapse) and no effect.
The success rate for full recovery (35.8 %) and improvement (25.0 %) is 60.8 %.
Diagram a) Study 14
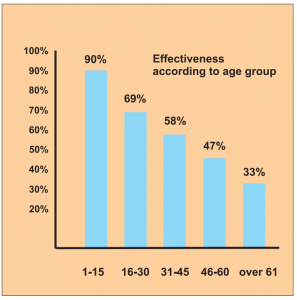
It is interesting to note the breakdown into age groups, where the efficacy rate in the 1 to 15-year-old patients is the highest at 90 %, followed by the 16 to 30-year-olds at around 69 %.
Diagram b) Study 14
This study has a level of evidence 5.
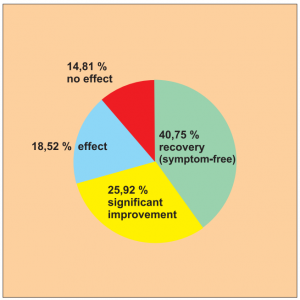
Study 15:
Single group cohort study with 54 patients across all age groups with nettle rash (urticaria), carried out by Zhang X. et al.
The success rate for this study is 66.67 % (40.75 + 25.92), as can be seen in the following diagram.
Extract from the biometric/medical assessment: Again we have a single group cohort study with defined efficacy criteria. The design is again similar to the usual observational studies used in Germany based on the conclusions described for the work of Du X. et al. The study is sufficiently representative with 54 patients taking part. As the authors themselves concede, the study is not adequate for long-term assessment.
Diagram Study 15
Literature:
The studies were carried out and published by:
- P. Schumacher: Biophysical Treatment of Allergies, Handbook on natural healing processes and specific trends in therapy, Published by O. Ausserer, ZDN series, Alfred & Söhne, Meran, 1992, pp 139 144; and Sonntag-Verlag, Stuttgart, 1994, pp 125-133;
- Yang Jinzhi and Zhang Li, Research Centre of the Jinan Paediatric Clinic in the province of Shandong for the Prevention and Treatment of Asthma; official translation from Maternal and Child Health Care of China, 2004, CN 22-1127/R;
- Yuan Ze, Huang Jioh, Wang Haiyan and Y. Chunyan. Department of Paediatrics of Xian, Central Hospital Hi’an: Original publication in Chinese with translation from Maternal and Child Health Care of China, 2004;
- Ze Y. and Haiyan W, Paediatrics of Xi’an Central Hospital, China: Clinical Results with the BICOM 2000 Bioresonance Device, paper from Medical Association Congress on BICOM® Therapy in Fulda, April/May 2005;
- J. Hennecke: Energetic Allergy Therapy – Possibilities and Experiences with BICOM® Bioresonance Therapy;
Medical Journal of Naturopathy 35 (1994) 427-432; and Two Years’ Experience with Allergy Therapy without Abstinence – Evaluation of a Statistical Study,
Practical Implications: International Medical Association Colloquium on BICOM® Therapy from 1 to 3 October 1993 in Fulda; - R. Machowinski and I. Gerlach: Prospective, randomised studies to assess the success of treatment using the patient’s own oscillations (BICOM) in cases of liver cell damage, paper given at the symposium in Fulda on 16.04.1996;
- B. Papez and Barovic, Maribor Teaching Hospital, Slovenia, Medical Rehabilitation Dept., led by Prim. Dr. sci., Dr. med. Zmago Turk: Report on the use of BICOM resonance therapy in cases of overstrain syndrome in high-performance athletes;
- O. Sakharov, D Lednyiczky, Kavetzky Institute for Experimental Pathology, Oncology and Radiobiology at the State Academy of Sciences, Ukraine: Summary representation of in-vitro modulation of phagocyte activity of human polymorphonuclear leucocytes by BICOM resonance therapy, Scientific Studies, Institut für Regulative Medizin, 1999;
- D. Sakharov, Z Savtsova et al: Investigation into the reconstitution of the immune system of radioactively contaminated mice by means of BICOM resonance therapy; Scientific Studies, Institut für Regulative Medizin, 1999;
- E. Giannazo, S. Valenti, D. Puzzo, Physiology Dept., Chair of Biophysics at the University of Catania; Research report 2002;
- Du Xia, Liu Yuanxia, Yang Jinzhi: Clinical observation of 79 cases treated for allergic skin conditions by means of bioresonance; Chi. Journal of Practical Medicine, Vol. 4, No. 3, March 2005 (official translation);
- Y. Feng et al: The clinical observation of the healing effect using the bioresonance therapy device in 150 cases of childhood allergy, Chin. Journal of Contemporary Paediatrics, Vol 7, No. 3, June 2005 (official translation);
- Huang Shuiming, Sun Zhangping, Fang Yucai: Clinical observation of the treatment of allergies and bronchial asthma in children with the bioresonance therapy device; Zhejiang Medical Journal (official translation), Edition 6/Volume 27/2005;
- Xu Minhong et al: Clinical observation of the treatment of chronic nettle rash using the bioresonance therapy device, China Journal of Leprosy and Skin Disease, Volume 21, No. 7, July 2005 (official translation);
- Zhang Xinlian, Wang Wenjie, Liu Qiang: Clinical observation of 54 cases treated for nettle rash using the BICOM bioresonance therapy device: China Scientific and Technical Journal, China Academic Journal (official translation), Volume 21, No. 8, August 2005;P-29/EVI-DE/August 06 0066; 08/06
Int. Med. Arbeitskreis BICOM Resonanz-Therapie (IMA BRT) Hans-Cornelius-Straße 4 D-82166 Gräfelfing Telefon 0 89/8 54 61 02 Telefax 0 89/8 54 61 03


