Alberto Foletti 1,3 , Settimio Grimaldi 1, Antonella Lisi1,2,3, Mario Ledda1,2,3 & Abraham R. Liboff2
1Institute of Translational Pharmacology – CNR, Rome, Italy, 2Department of Physics, Oakland University, Rochester Hills, MI, USA, and 3Department of Innovative Technologies-DTI, University of Applied Sciences of Southern Switzerland-SUPSI, Manno, Switzerland
Only recently has the critical importance of electromagnetic (EM) field interactions in biology and medicine been recognized. We review the phenomenon of resonance signalling, discussing how specific frequencies modulate cellular function to restore or maintain health. The application of EM-tuned signals represents more than merely a new tool in information medicine. It can also be viewed in the larger context of EM medicine, the all-encompassing view that elevates the EM over the biochemical. The discovery by Zhadin that ultrasmall magnetic intensities are biologically significant suggests that EM signalling is endogenous to cell regulation, and consequently that the remarkable effectiveness of EM resonance treatments reflects a fundamental aspect of biological systems. The concept that organisms contain mechanisms for generating biologically useful electric signals is not new, dating back to the nineteenth-century discovery of currents of injury by Matteucci. The corresponding modern-day version is that ion cyclotron resonance magnetic field combinations help regulate biological information. The next advance in medicine will be to discern and apply those EM signalling parameters acting to promote wellness, with decreasing reliance on marginal biochemical remediation and pharmaceuticals.
Keywords: Bioresonance, coherence domains, electromagnetic medicine, information medicine, ion cyclotron resonance, stem cell differentiation, water structure
Introduction
Although the question of cell signalling is regarded as fundamental, it is usually thought of in a molecular manner, a prime example being the second messenger role played by the calcium ion. An enormous number of downstream effects are connected to this concept. These are rarely if ever, framed in electric terms. But the experimental record of the past 40 years makes it impossible to dismiss the underlying electromagnetic (EM) nature of cellular signalling. The presently accepted model of calcium as a second messenger is incomplete because it fails to take note of the electrical signal that initiates and is part of the process.
Attempts to study cell function as a problem in electrical engineering have not been fruitful. When asking how much useful information can be transmitted in a biological system, some observers simply apply Shannon’s theorem to neural elements in the same way as one would to cables, utilizing bandwidth, the number of different frequencies that can simultaneously be transmitted as the key variable. The question reduces to: how well can one receive biologically useful information when the bandwidth is very narrow? This problem, first studied by Weaver and Astumian (1990) and. Adair (1991), adopted an approach from electrical engineering, where information transfer is limited by the level of thermal noise to be expected in a circuit element of resistance R and bandwidth zu at temperature T, using the expression (817)2 = 4kRTAv. However, numerous objections have been raised (Bier, 2005; Vincze, Szasz, & Liboff, 2008; Vincze, Szasz, & Szasz, 2005) to this simplistic application to living tissue, first, because improper values were assigned to the resistance, capacitance and bandwidth, and second, because living tissue is itself actively involved and cannot be treated as a passive circuit element.
Increasingly, it is recognized that life may have an EM basis, either in terms of the local field or potential-dependent functions (Bersani, 1999; Presman, 1970) or more holistically (Liboff, 1994) as a description that complements, if not supplants, the usual biochemical paradigm. We adopt the view that regards life as an expression of the EM field.
Many have speculated in this subject, searching for a role and for a meaning of the presence of EM signals inside and around cells, tissues and living organisms. It is now well known that EM activity can be detected at the level of cell membranes (Becker & Bachman, 1965; Becker & Brown, 1965; Hoelzel & Lamprecht, 1994, 1995; Jelinek et al., 1999; Pelling, Sehati, Gralla, Valentine, & Gimzewski, 2004, 2005, 2007; Pohl, 1980, 1981; Rapp, 1979, 1980) and at the level of microtubules (Pokorny, 1999, 2001; Pokorny, Jelinek, & Trkal, 1998) particularly. One important, recently discovered phenomenon, a resonance effect, is involved in generating communication signals by transferring highly specific and functional information at certain frequencies and intensities. Experiments demonstrate that the use of specific EM signals in the extremely low-frequency (ELF) range can trigger specific biological pathways. Equally important, the amount of useful energy transfer involved in these studies is so low that we must assume that this phenomenon is specifically designed as an information effect, for purposes of control. It is clear that the earlier approach of treating EM effects in living things as an exercise in electrical engineering is overly simplistic and without merit.
We shall use the term Bioresonance to describe the situation when resonance signalling occurs within a biological structure such as a specific part of a cell or a tissue or within a living organism as a whole. One type of Bioresonance, currently under intense study (Pokorny, 2001), hypothesizes that endogenous high-frequency signals derived from microtubular generating structures may constitute a form of resonance engineering, acting to maintain cellular homeostasis. Another type of Bioresonance is the selective low-frequency EM stimulation of one or more biological cations at cyclotron resonance (ICR) conditions that are determined by their individual charge-to-mass ratios (Liboff, 1985). ICR stimulation has been widely observed, occurring exogenously as the result of externally applied electric field or magnetic field (MF) and very likely endogenously as well, because of self-generated electric fields at the cell membrane.
EM medicine
To many in the medical community, the term EM medicine refers to a small group of diagnostic applications, the foremost example being magnetic resonance imaging (MRI). Much less attention has been paid to EM therapeutic applications, which have been increasingly shown to be useful in treating widely disparate problems, ranging from bony nonunions to chemotherapeutic stress disorder (Diebert, McLeod, Smith, & Liboff, 1994; Rossi, Corsetti, Sukkar, & Poggi, 2007). Various other types of therapies have reported efficacious clinical results (Brugemann, 1990; Islamov, Balabanova, Funtikov, Gotovskii, & Meizerov, 2002; Islamov, Funtikov, Bobrovski, & Gotovskii, 1999). In addition to the clinical aspects, there have been important advances in the biology of Bioresonance. These include striking resonance effects on amino acid solutions and eukaryotic cell models and human cell lines (Giuliani et al., 2008; Fitzsimmons, Ryaby, Mohan, Magee, & Baylink, 1995; Rozek, Sherman, Liboff, McLeod, & Smith, 1987; Zhadin, Novikov, Barnes, & Pergola, 1998). Most excitingly, we now understand that Bioresonance applied to human stem cells is able to generate modifications in well-defined cells and strongly affect differentiation processes.
It is important to understand that none of this work in resonance signalling, even the now decades-long success with bone repair, can be explained using biochemical processes, beyond knowing that these processes are somehow set into motion by resonance fields. In the eyes of bioscientists, this inability to provide a “classical” explanation makes this research suspect. The clinical concept of EM medicine is difficult to accept because of the belief in the century-old paradigm founded by Pasteur and Koch and elaborated by Watson and Crick. To most clinicians, it is inconceivable that there are other avenues to wellness and longevity than those presently followed and explained biochemically, in terms of molecular biology. At most, EM medicine is regarded as a convenient technological adjunct to diagnostic medicine, providing useful tools such as MRI, electro- and magnetocardiography and electromyography that enable the clinician to further explore patient problems, but always in the context of existing medical understanding. Where EM therapy has proven useful, explanations are attempted with links to physical variables such as heat that can be more readily related to the current physiological paradigm.
But experiments show that there is another side to the clinical use of EM, particularly as regards the application of very weakly intense low-frequency EM signals. Cells are profoundly affected by MFs that are far too weak to be explained by thermal effects. The question then arises by what means are meaningful biochemical changes in living things affected by EM fields if these changes are accompanied by no measurable changes in heat or chemical energy?
We argue that electromagnetism provides an alternate route to molecular biology that can lead to improved medical understanding and efficacious application. It is clear that EM applications can be used to treat illness following pathways that are outside present clinical biochemical regimens. EM medicine, in its most general realization, makes use of interactions that in some cases even go beyond resonance signalling. Some of these EM therapies that do not involve ICR stimulation include repetitive transcranial magnetic stimulation (rTMS) (Figiel et al., 1998; George et al., 1995) to treat depression, EM-induced hyperthermia (Andocs, Szasz, & Szasz, 2009) and electrochemical treatment for late-stage cancers (Chou, McDougall, Ahn, & Vora, 1997) and application of electric fields at hundreds of kilohertz to treat aggressive glioblastoma and lung cancer (Kirson et al., 2007, 2009). Of these, only rTMS has to date gained medical approval in the USA.
Effects on cell function
For some decades, evidence has accumulated that electromagnetism plays a key, albeit poorly understood, role in physiological outcomes (Aarholt, Flinn, & Smith, 1981; Adey & Bawin, 1982; Adey, Bawin, & Lawrence, 1982; Astumian & Robertson, 1989; Astumian, Weaver, & Adair, 1995; Ayrapetyan, Grigorian, Avanesyan, & Stamboltsian, 1994; Barnes, 1996; Basset, 1993; Basset, Pawluk, Pi11a, 1974; Eichwald Sz Kaiser, 1993; Eichwald & Walleczek, 1996a,b, 1997, 1998; Kaiser, 1996). There is now broad acceptance that cell function is robustly sensitive to specific electric and magnetic signals. Studies have confirmed the reality of the effects of low-frequency MFs on cell characteristics, including membranes, calcium transport, cell proliferation and chromatin conformation.
Electrical properties such as membrane surface charge and potential are especially influenced by weak low-frequency MFs (Blackman, Benane, & House, 1993a,b; Blackman, Benane, House, & Elliot, 1990; Blackman, Kinney, House, & Joines, 1989; Blank 8/ Soo, 1992, 1993a,b; Markin & Tsong, 1991a,b,c; Tsong, Liu, Chauvin, & Astumian, 1989; Tsong, 1990, 1992; Vincze et al., 2005). Lisi, Ledda, et al. (2006) investigated whether exposure to an ELF EM field can affect the molecular biology of pituitary gland-derived AtT20 cells. When continuously exposed to a 2 mT low-frequency EM field, a statistically significant increase in intracellular calcium and membrane depolarization was found. Additional morphological changes, as well as rearrangements in actin filament distribution occur in the plasma membrane.
ELF EM fields can induce depolarization in the cell membrane followed by an increase in [Ca21i and an expression of neurofilament protein. Santoro et al. (1997) reported a decrease in membrane fluidity and reorganized cytoskeletal components after exposure to ELF MF in human B lymphoid (Raj° cells. ELF MFs can influence the structure of protein molecules within the biomembrane and also affect the expression of relevant genes and proteins (Baker, Spadaro, Marino, & Becker, 1974; Baker, Becker, 81 Spadaro, 1974; Becker, 1967; Becker, 1972; Becker, 1987, 2002; Becker, Chapin, & Sherry, 1974; Becker & Murray, 1970; Becker & Spadaro, 1972; Berg, 1993; Liboff & Jenrow, 2002).
Furthermore, MFs have been shown to stimulate proliferation and differentiation of neuronal cells in culture (Blackman, Blanchard, Benane, & House, 1994; Smith, Liboff, & McLeod, 1992). There is a large body of work (Cossarizza, Monti, Bersani, Cadossi, & Sacchi Franceschi, 1989; Cossarizza, Monti, Bersani, Paganelli et al., 1989; Cossarizza et al., 1993; Grimaldi et al., 1997; Liboff, Rozek, Sherman, McLeod, & Smith, 1987; Lyle et al., 1991; Ross, 1990; Walleczek, 1992; Yost & Liburdy, 1992) establishing the response of human lymphocytes to low-frequency MFs. Liburdy (1992) detected an increase in calcium uptake into mitogen-stimulated rat thymocytes and human lymphocytes during exposure to a 60-Hz ELF field. These and other well-documented effects include altered proliferative response to mitogens as well as changes in calcium uptake and signal transduction. Studies by Belyaev, Alipov, and Harms-Ringdahl (1997), Belyaev and Alipov (2001), Sarimov, Alipov, and Belyaev (2011) greatly reinforced this conclusion, determining that chromatin conformation in human lymphocytes is sharply affected by exposure to weak low-frequency MFs.
ELF resonance fields stimulate embryonic stem cell differentiation into cardiomyocytes by triggering the expression of specific cardiac lineage-promoting genes (Gaetani et al., 2009; Lisi, Ledda, De Carlo, Pozzi, et al., 2008). Cardiac stem cells (CSCs) from human endomyocardial biopsy specimens and mesenchymal stem cells can self-assemble into multicellular clusters known as cardiospheres (CSps) that engraft and partially regenerate infarcted myocardium. CSps and CSp-derived cells were exposed for 5 days in a magnetically shielded room with the cells exposed simultaneously to a weak magnetostadc field and an alternating (AC) low-frequency (7 Hz) MF, close to the cyclotron frequency corresponding to the charge-to-mass ratio of the Ca2+ ion. Cardiac markers were upregulated and angiogenic markers downregulated. In short, exposure to 7 Hz calcium ICR MF combinations can modulate the cardiogenic versus angiogenic differentiation process of ex vivo expanded CSCs. As discussed below, this may pave the way for novel approaches in tissue engineering and cellular therapy.
The role of cytosolic Ca2+ has long been recognized in the regulation of cellular and molecular interactions. Signal transduction related to Ca2± oscillations can provide molecular cues for cell functions such as differentiation and proliferation. Although Ca2+ dynamics are versatile and likely to depend on the cell type, their role in human CSC differentiation as revealed through resonance signalling is yet to be fully elucidated.
The underlying science
The mid-1980s was marked by the discovery by Blackman and Liboff of a surprising phenomenon (Blackman, Benane, Rabinowitz, House, & Joines, 1985; Liboff, 1985): a low-frequency AC MF is capable of changing free calcium concentrations in brain tissue, but only in the presence of a parallel simultaneously applied static [[}Cl MF. The most prominent effect was observed at the AC field frequency close to the cyclotron frequency of the calcium ion. Most subsequent ICR cellular studies have focused on the Ca2+ ion. As a second messenger, it is involved in regulation at all stages of cellular growth and development, including proliferation and differentiation, and in the assembling and disassembling of cytoskeleton elements.
A confirmation that the charge-to-mass ratio was explicitly involved in this effect was obtained when Liboff substituted isotopic 45Ca for “Ca in a study on lymphocyte proliferation, showing that the frequency where the maximum ICR effect on proliferation occurred was shifted down by a factor of 12%, exactly what is to be expected for a change of mass of 5 parts out of 40 (Liboff et al., 1987). The angular cyclotron frequency is defined as co =- (q1m)130, where q and m are the charge and mass of the ion, and Bo is the DC field. This work in ICR opened a new line of research in studying the interactions between EM fields and living things.
There were three unexpected aspects to this phenomenon: (1) the validity of Lorentz law and the consequent resonance effects in biological systems, (2) profound physiological changes following application of AC and DC MFs tuned to cyclotron frequency resonance conditions and (3) the remarkable effectiveness of very small flux density values of the applied MFs (at first), measured in tens of microtesla, and ELFs of AC MFs, measured in tens of hertz or less. Because these effects appeared to violate simplistic analyses involving magnetic induction, these results evoked much suspicion in the scientific community. However, many subsequent confirmations, performed on different model systems in diverse experimental situations, proved that these weak low-frequency effects are indeed real. It has since become clear that MF combinations, when tuned at ICR, act to regulate the flow of biological information.
There are now dozens of examples from widely different model systems showing that exposures to ICR combined MFs can result in profound physiological changes, not only in animal systems (Smith, Liboff, & McLeod, 1991; Smith, McLeod, Liboff, & Cooksey, 1987; Thomas, Schrot, & Liboff, 1986) but also in plants (Galland & Pazur, 2005; Liboff, 2006). Early experiments verifying this phenomenon were nearly always performed by looking for these changes under separate applications of the DC field alone or the AC field alone, as well as for combined fields. It was repeatedly observed that changes only occurred for combined AC and DC MFs.
As little as a 10-minute exposure to Ca2+ ICR fields will stimulate insulin-like growth factor II and DNA synthesis in human osteosarcoma bone cells (Fitzsimmons et al., 1995). A 1-hour Ca2* ICR field applied to thymic lymphocytes in culture will inhibit calcium influx triggered by the mitogen Concanavalin A (Yost & Liburdy, 1992). The same ICR field application, again on human lymphocytes in culture, is antagonistic to nifedipine, the pharmaceutical employed extensively as a calcium channel blocker (Rozek et al., 1987).
In addition to these and other Ca2+ ICR studies, consistent effects have also been reported for K± and Mg2± cyclotron resonance MF applications (Liboff, 2006). ICR fields tuned to Zn2+ significantly inhibit cell proliferation, not only in VH-10 human fibroblasts but also, more importantly, in two malignant lines, HeLa cervical cancer and p53 His-273 osteosarcoma (Sarimov, Markova, Johansson, Jenssen, & Belyaev, 2005). The resonance treatment of both bony nonunions and spinal fusion employs the simultaneous application of both Ca24 and Mg2+ ICR fields (Diebert et al., 1994). Most recently, two resonance frequencies have been applied simultaneously, in the one case to treat (mouse) ascites caused by cancer and in the other to dissolve Alzheimer’s plaque, both with effective results (Bobkova, Novikov, Medvinskaya, & Fesenko, 2005; Novikov, Novikov, & Fesenko, 2009).
The inescapable conclusion from this long list of reports is that the ICR mechanism, whatever its molecular basis, is of enormous biological significance. We are able to make reproducible and consistent physiological changes of various sorts in the widest imaginable range of genera simply by applying weak MFs tuned to the charge-to-mass ratio of various biological cations. It is very clear that these ICR applications must be part of a heretofore unknown system that carries physiological information/instructions, and that better understanding will open the way to providing a radically new means of controlling wellness.
Studies on the effects of ICR applications have also been extended in a number of fascinating directions, including the discovery by Zhadin that ICR fields, when tuned to the charge-to-mass ratios of polar amino acids, greatly enhance their aqueous conductivity (Zhadin et al., 1998). In turn, this work led to an increased interest in realizing ICR effects at remarkably small MFs, of the order of 50 nT, and also to the likely involvement of MF-induced changes in water structure as the underlying reason for this strange low-field, low-frequency ICR phenomenon.
Following Zhadin’s approach (Zhad,in et al., 1998), Giuliani and Grimaldi studied the influence of combined DC and AC parallel MFs on the current through an aqueous solution of glutamic acid (glu) (Giuliani et al., 2008). The results demonstrated for the first time that, for combined DC and AC parallel MFs matching the ICR of a particular charged molecule in biological tissue, an intrinsic weak MF is generated by coherent ion currents in the cell.
It has been difficult to understand the underlying physical mechanisms of these resonance effects. Liboff considered the transport of ions in membrane-bound channels under the action of ICR-tuned combined MFs, suggesting a mechanism similar to the helical motion of charged particles in free space under the influence of the Lorentz force (Figiel et al., 1998; Liboff, Smith, & Mc Leod, 1995; McLeod & Liboff, 1986). But at body temperature, this idea can be realized only at low densities and by including a much larger radius of ionic motion. The idea that parametric resonance might be responsible for such effects is also not very fruitful for lack of a necessary low-frequency harmonic oscillator in cellular systems (Blackman, Blanchard, Benane, & House, 1995, 1999; Blanchard & Blackman, 1994; Lednev, 1991; Vincze et al., 2008). Models employing Larmor precession and other classical approaches have also not proven helpful (Zhadin, 1996, 1998, 2001; Zhadin & Barnes, 2005; Zhadin & Fesenko, 1990; Zhadin & Giuliani, 2006).
In the most recent attempt to find a reasonable explanation, Preparata’s work in quantum electrodynamics (QED) (Preparata, 1995) was used to show that liquid water can be viewed as being in an equilibrium state between coherent and incoherent components. The coherent component is contained within spherical “coherence domains” (CDs) in which all molecules synchronously oscillate with the same phase. CDs are surrounded by the incoherent component where molecules oscillate with random phases (Del Giudice, Galimberti, Gamberane, & Preparata, 1995; Del Giudice & Preparata, 1995). At room temperature, the total volume of such domains is about 40% of the total. The existence of CDs in water has been demonstrated in a set of experiments on pure water exposed to high voltages (Fuchs et al., 2007; Giuliani et al., 2009). Under this condition, the electric field concentrates inside the water, arranging the water molecules to form highly ordered structures.
Because the ions in the immediate vicinity of a structured water domain are in a region where there is total absence of friction, the effects due to viscosity are drastically reduced, which in turn makes the effects of Lorentz forces more likely. An ion trapped around a water CD can behave as if it were in a vacuum. Thus, if a DC uniform MF is applied, the ion will move in a circular path around the Cl]. If an appropriate additional AC MF is applied with the same frequency of the ion circular frequency (ion cyclotron frequency), the ion will be removed from orbits around the CD and it can jump into the noncoherent water area and become biologically available. Therefore, the ICR problem appears to be solved, at least in part, using the QED approach initiated by Preparata and extended by Del Giudice.
This explanation has been supported by experiments performed in different laboratories studying the behavior of glu at the glutamate ICR condition. Glu solutions in an electrolytic cell were irradiated under controlled conditions for a range of ELFs and the current flowing in the electrolytic cell continuously recorded. When the resonant condition for the charge-to-mass ratio in glu was reached (4.1 Hz), a discrete peak in DC current was recorded (Giuliani et al., 2008).
Clinical applications
There is potentially a wide diversity to the clinical applications of (nonthermal) resonance signalling. The earliest example dates back to the 1980s when it was applied to the repair of bony nonunions and as an adjunct to healing following spinal surgery. This application used either pulsed MF (PMF) (Basset et al., 1974) or ICR (Diebert et al., 1994), both techniques approved by the US Food and Drug Administration. The PMF approach applies pulses with a repetition rate of 15 Hz, which may be the actual source of the biological stimulation. In the ICR application, MFs tuned jointly to Ca2+ and Mg2+ are applied for 30 minutes per day for weeks at a time. Very significant responses are consistently observed (often providing a noninvasive alternative to amputation), at success rates approaching 80%.
Resonant MF combinations tuned to amino acid charge-to-mass ratios were used by Novikov to hydrolyze proteins, notably p-amyloid, one of the key markers for Alzheimer’s disease (Bobkova et al., 2005). This result, as with many other ICR experiments, illustrates the difficulty in reconciling resonance signalling with conventional biochemistry. The energy supplied by the MF in this work was orders of magnitude less than that required for protein hydrolysis. Using a similar ICR field, Novikov was also able to significantly extend survival rates in mice infected with Ehrlich ascites tumors (Novikov, Novikov, & Fesenko, 2009).
A very different approach to ICR medical therapy is found in the Seqex device, which applies an oscillating MF to the patient’s entire body while simultaneously taking advantage of the local parallel vertical component of the earth’s MF to achieve resonance. It has been effectively used to treat the debilitating depression that often accompanies chemotherapy following cancer remediation (Raggi et al., 2008; Rossi et al., 2007). In addition to the fact that this device employs holistic application of the combined fields, it is unique in that the applied ICR frequency is not calculated from ionic charge-to-mass ratios, but is determined by first finding in a prior separate evaluation the specific frequency conditions that sharply alter the whole-body bioimpedance. Once determined, this frequency information is stored on a “smart card” for future treatments on that patient. It is worth noting that the change in whole-body bioimpedance at resonance is consistent with the sharp changes in ionic conductivity that were observed by Zhadin et al. (1998), Giuliani et al. (2008), Pazur (2004) and Alberto et al. (2008).
The most recent clinical application of resonance signalling may also be the most spectacular. Probably the most difficult aspect in treating heart failure is the inability of the damaged heart muscle to regenerate, the reason why surgeons are often forced to resort to heart transplants. One objective of stern cell therapy is to provide working replacement of infarcted heart muscle with suitably differentiated heart cells.
The usefulness of the Bioresonance concept to this important current clinical problem was demonstrated by studying the effect of combined DC and AC MFs tuned at Ca2+ ICR on a system of human CSCs (Gaetani et al., 2009). It was thought that suitable combinations of EMFs might affect intracellular Ca2+ levels, triggering progenitor cell proliferation and differentiation, a reasonable approach, considering that Ca2± ions are essential regulatory components of all organisms. On the basis of previous results obtained with other cellular mode)s, CSCs were exposed for up to 5 days to the ICR frequency corresponding to the charge-to-mass ratio of the Ca 2+ ion.
These exposures produced several interesting effects. CSCs exposed to ELF EMF had a higher metabolic activity compared with unexposed cells. This can be related to an increase in cell proliferation, as was evidenced by BrdU incorporation curves. This trend was reduced after 3 days of exposure, perhaps due to contact inhibition and/or the beginning of the differentiation process, well documented after 5 days in CSCs at transcriptional and translational levels. Usually, proliferation and differentiation are considered mutually exclusive paths, but since CSCs represent heterogeneous populations of progenitor cells at various stages of commitment, one could expect slightly different responses to proliferative and differentiation stimuli at each intermediate stage. To a certain extent, these responses possibly overlap the progressive maturation process of the entire progenitor population.
The increase in mRNA levels of cardiac-specific markers, demonstrated by real-time polymerase chain reaction, was associated with an increase in the corresponding protein expression. Although CSCs spontaneously differentiate toward the cardiogenic phenotype, this process was improved by ICR exposure. The improvement in the differentiation process was cardiac specific, although not terminal. After Ca2+ ICR exposure, cardiac markers were upregulated, while vascular markers were either unaffected or reduced. Cardiac-specific differentiation was further evidenced when mRNA levels of cardiac markers of exposed and unexposed cells were compared with those of adult heart tissue from biopsy.
The reduction in the expression ratios of heart tissue versus the Ca2+ ICR exposed compared with unexposed samples represents a different and effective means to indicate cardiac differentiation. Confocal analysis confirmed an increase in the expression of cardiac markers. Altogether these results suggest that a lineage-specific differentiation is driven by the exposure to Ca ICR Bioresonance signals.
It is important to understand that the same experiments repeated at other frequencies not matching ICRs of biologically relevant ions did not display any significant effect at the transcriptional level, endorsing the hypothesis that these results were specifically Ca2+ mediated. This study demonstrated unequivocally an increased intracellular calcium accumulation in CSCs after chronic exposures to Ca2± ICR, and the interesting observation that this is directly related to information transfer between mitochondria and the cytosol.
Therefore, the modulation of both proliferation and cardiac differentiation observed in Ca2+ ICR exposed cells correlates to induced changes in intracellular Ca2+ accumulation and mobilization, potentially modulating signal cascade pathways. Independent of the involved mechanisms, the induced differentiation toward the cardiac phenotype has relevant implications for the use of CSCs in tissue engineering and cell therapy. The modulation of cell proliferation and specific differentiation elicited by this system, a result of EM exposure at ICR frequency, may represent an effective, minimally manipulative and safe biotechnological tool to improve patient cardiac regenerative potential.
Conclusions
The Prometheus myth is a fitting model for regenerative medicine. As punishment for giving fire to humanity, Zeus ordered Prometheus chained to a rock and sent an eagle to eat his liver each day. However, Prometheus’ liver was able to regenerate itself daily, during nighttime, enabling him to survive. Today we hope to make the legendary concept of regeneration into reality by developing therapies to restore lost, damaged or ageing cells and tissues in the human body. It has been shown that ICR differentiation of human adult CSCs is feasible. Thus, it appears that ICR Bioresonance techniques may be extremely useful in advancing the implementation of regenerative medicine and tissue engineering.
But, the clinical potential for resonance signalling is not limited to organ repair and regeneration. Consider, for example, one interesting observation found in ICR studies. In more than a few reports, merely changing the resonance condition from one ion to another resulted in an opposite response. This phenomenon was first observed by Smith in his studies on diatom motility (Baker, Becker, et al., 1974) and later reported by others (Becker, 2002; Belova & Lednev, 2000; Liboff, 2002; Regling, Brueckner, Kimura, & Liboff, 2002; Smith, McLeod, & Liboff, 1993, 1995;
Table 1. Ionic tuning can drastically alter physiological outcome.
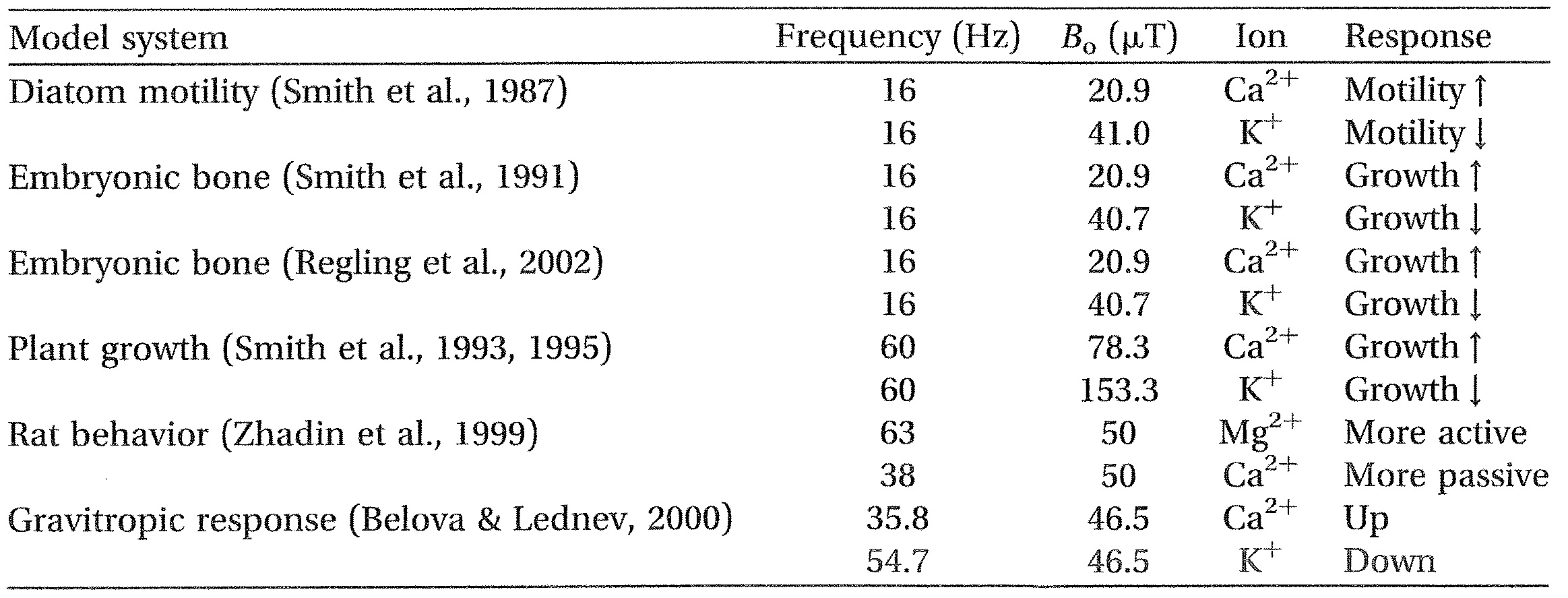
Note: This effect is observed for different magnetostatic fields at the same resonant frequency or, equivalently, for different frequencies at the same static magnetic intensity.
Zhadin, Deryugina & Pisachenko, 1999) (Table 1). Liboff (2010) suggested that this effect likely reflects the endogenous nature of Bioresonance, wherein multiple ionic resonances occur simultaneously giving rise to a balanced homeostatic physiologic outcome. If this is true then it should be possible, in principle, to selectively reduce the undesirable in favour of the desirable. Reports (Smith et al., 1992) indicate that ICR applications can increase the rates of proliferation in neuroblastoma cell culture. Is it possible that there exist ICR conditions that would have the opposite effect, namely to reduce the rates of proliferation in cancer cell lines, opening the way to new cancer-fighting techniques?
Since the time of Galvani, evidence has accumulated indicating that living systems make use of EM fields. Until recently, this connection between electricity and biology was limited to the nervous system. It now appears that all the major functional structures of living systems can be regarded as EM field sources. Organisms, in turn, can be thought of as EM objects containing atomic and molecular structures.
Medical use of EMF has a long history. Modem medical applications of EMF are used to heal nonunions of bone fractures (Zhadin et al., 1999) and treat some bone-related diseases (e.g. osteoporosis and osteoarthritis), although the specific molecular mechanisms are not understood. The application of EMF to stimulate osteogenesis, for instance, is incorrectly based on the idea of stimulating the natural endogenous streaming potentials in bone. Although EM medicine is still in its infancy, there is much evidence that ICR exposure can tune eukaryotic cells toward cell differentiation and maturation, influencing physiological processes (Berg & Zang, 1993; Bistolfi, 1987; Bistolfi, 1990; Lisi, Foletti, et al., 2006; Lisi, Ledda, De Carlo, Foletti, et al., 2008; Lisi, Rieti, et al., 2006). These data suggest possible future applications of EM protocols, including Bioresonance, for the treatment of a wide range of human diseases by means of specific relevant frequency patterns delivered with specific biomedical technology, thereby providing an important tool in clinical medical practice.
In a certain sense, endogenous resonance signaling represents a more modern version of Carlos Matteucci’s nineteenth-century work on currents of injury (Matteucci, 1834), rescued from obscurity by Robert Becker, who recognized these currents as an intrinsic feature of living things, a built-in ability that uses electricity to regulate and repair the organism (Becker & Spadaro, 1972). ICR signalling can be regarded as a cellular version of Matteucci’s currents of injury.
Apart from its obvious potential as a tool in medicine, there is a deeper question attached to the phenomenon of Bioresonance. What is the underlying biological reason for applications of ICR magnetic stimulation to result in such robust and varied effects, ranging from enhanced cell proliferation to stem cell differentiation, from changing the rates of plant growth to altering the conductivity of glu solutions? This speaks of the likelihood of heretofore unknown biological effects that still remain to be illuminated. One possibility that has been recently hypothesized (Liboff, 2010) is that these various ICR experiments, all involving time-varying MFs, are actually tapping into a basic biological process utilizing time-varying electric fields (Liboff, 1997) stochastically generated at the cell membrane to provide a regulatory system for cell function involving the geomagnetic field. Still another possibility surrounds the remarkable response of lymphocytes to weak ELF MFs, suggesting that the immune system is responsive to EM signals generated by pathogens (Liboff, in press). Both speculations fall within the guiding hypothesis of this work, namely that ELF signaling is an important endogenous feature of living organisms.
We still need to build bridges between biology and physics, particularly QED (Del Giudice, Fleischmann, Preparata, & Talpo, 2002; Del Giudice & Preparata, 1995; Del Giudice et al., 1995; Preparata, 1995), information theory (Foletti, Lisi, Ledda, De Carlo, & Grimaldi, 2009; Rubik, 1995; Stuart, 1985a,b) and the theory of open systems (Barbieri, 2004; Becker, 2004; Bertalanffy, 1949, 1950; Frohlich, 1988; Frohlich & Kremer, 1983; Liboff, 2004, 2007; Mae-Wan, 1998; Pokorny & Wu, 1998; Popp & Beloussov, 2003; Volodyaev, 2005; Zewail, 2008). In this framework, the concept of resonance signalling can play a unifying role in bridging this gap. Not only can it provide a new tool to help manage biological complexity at the bedside of the sick but perhaps it will also help us find an answer to the old question about the basis to life (Sahu, Hirata, Fujuita, Ghosh, & Bandyopadhyay, in press; Schrodinger, 1944; Darr, Popp, & Schommers, 2002).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
Aarholt E, Flinn EA, Smith CW (1981). Effects of low-frequency magnetic fields on bacterial growth rate. Phys Med Biol 26:613-621.
Adair RK (1991). Constraints on biological effects of weak extremely-low-frequency electromagnetic fields. Physical Rev A 43:1039-1048.
Adey WR, Bawin S (1982). Binding and release of brain calcium by low-level electromagnetic fields: a review. Radio Sci 17(5S):149S -157S.
Adey WR, Bawin FM, Lawrence AF (1982). Effects of weak, amplitude-modulated fields on calcium efflux from awake cat cerebral cortex. Bioelectromagnetics 3:295-308.
Alberto D, Buss° I, Crotti G, Gandini M, Garfagnini R, Giudici P, Gnesi I, et al. (2008). Effects of static and low-frequency alternating magnetic fields on the ionic electrolytic currents of glutamic acid aqueous solution. Electromagnetic Biology and Medicine 27:25-39.
Andocs G, Szasz 0, Szasz A (2009). Oncothermia treatment of cancer: from the laboratory to the clinic. Electromagn Biol Med 28:148-165.
Astumian RD, Robertson B (1989). Nonlinear effect of an oscillating electric field on membrane proteins. J Chem Phys 72:4891-4899.
Astumian RD, Weaver JC, Adair RK (1995). Rectification and signal averaging of weak electric fields by biological cells. Proc Natl Acad Sci USA 92(9):3740- 3743.
Ayrapetyan R, Grigorian K, Avanesyan A, Stamboltsian K (1994). Magnetic field alter electrical properties of solutions and their physiological effects. Bioelectromagnetics 15:133-142.
Baker B, Spadaro J, Marino A, Becker RO (1974). Electrical stimulation of articular cartilage regeneration. Ann N Y Acad Sci 238:491-499.
Baker B, Becker RO, Spadaro J (1974). A study of electrochemical enhancement of articular cartilage repair. Clin Orthop Relat Res 102:251-267.
Barbieri M (2004). The definition of information and meaning two possible boundaries between physics and biology. Riv Biol 97:91-109.
Barnes FS (1996). Effect of electro-magnetic field on the rate of chemical reactions. Biophysics 41:801-880. Basset CAL (1993). Beneficial effects of electromagnetic fields. J Cell Biochem 51:387-393.
Basset CAL, Pawluk RJ, Pilla AA (1974). Augmentation of bone repair by inductively coupled
electromagnetic field. Science 184:575-579.
Becker RO (1967). The electrical control of growth processes. Med Times 96:657-669.
Becker RO (1972). Stimulation of partial limb regeneration in rats. Nature 235:109-111,
Becker DO (1987). Electromagnetism and the revolution in medicine. Acupunct Electrother Res 12(1): 75 – 79.
Becker RO (2002). Induced dedifferentiation: a possible alternative to embryonic stem cell transplant. NeuroRehabilitation 17(1):23 -31,
Becker RO (2004). Exploring new horizons in electromedicine. J Ahern Complement Med 10(0:17-18. Becker RO, Bachman CH (1965), Bioelectric effects in tissue. Clin Orthop Relat Res 43:251-253, Becker RO, Brown FM (1965). Photoelectric effects in human bone. Nature 206(991):1325 -1328.
Becker RO, Murray DG (1970). The electrical system regulating fracture healing in amphibians. Clin
Orthop Relat Res 73:169-198.
Becker RO, Spadaro JA (1972). Electrical stimulation of partial limb regeneration in mammals. Bull N Y Acad Med 48(4):627-641.
Becker RO, Chapin S, Sherry R (1974). Regeneration of the ventricular myocardium in amphibians. Nature 248(444):145 -147.
Belova NA, Lednev VV (2000). Activation and inhibition of gravitropic response in plants by weak combined magnetic fields. Biophysics 45:1069-1074.
Belyaev IY, Alipov ED (2001). Frequency, -dependent effects of ELF on chromatin conformation in Escherichia co//coUa and human lymphocytes. Biophys Biochim Acta 1526:269-276.
Belyaev IY, Alipoy YD, Harms-Ringdahl M (1997). Effects of zero magnetic field on the conformation of chromatin in human cells. Biophys Biochim Acta 1336:465-473.
Berg H (1993). Electrostimulation of cell metabolism by low frequency electric and electromagnetic fields. Biolectrochem Bioener 31:1-25.
Berg H, Zang L (1993). Electrostimulation in cell biology by low frequency electromagnetic fields. Electro Magnetobiol 12(2):147- 163.
Bersani F, editor. (1999). Electricity and magnetism in biology and medicine. New York: Klu e Academic/ Plenum Publishers.
Bertalanffy Lyon ()949), Open systems in physics and biology. Nature 163:384.
Bertalanffy Lyon (1950). The theory of open systems in physics and biology. Science 111:23-29.
Bier M (2005). Gauging the strengths of power frequency fields against membrane electrical noise. Bioelectromagnetics 26:595-609.
Bistolfi F (1987). Classification of possible targets of interaction of magnetic fields with living matter. Panminerva Med 29(1):71 -73.
Bistolfi F (1990). The bioelectronic connectional system (BCS): a therapeutic target for nonionizing radiation. Panminerva Med 32(1):10 – 18.
Blackman Cr, Benane SG, Rabinowitz JR, House DE, Joines WT (1985). A role for the magnetic field
in the radiation-induced efflux of calcium ions from brain tissue in vitro. Bioelectromagnetics 6:327-337. Blackman CF, Kinney LS, House DE, Joines WT (1989). Multiple power-density windows and their possible
origin. Bioelectromagnetics 10:115-128.
Blackman C, Benane S, House D, Elliot D (1990). Importance of alignment between local DC magnetic field and an oscillating magnetic field in response of brain tissue in vitro and in vivo. Bioelectromagnetics 11:159-167.
Blackman CF, Benane S, House D (1993a). Evidence for direct effect of magnetic fields on neurite outgrowth. FASEB J 7:801-806.
Blackman CF, Benane S, House D (1993b). Frequency-dependent interference by magnetic fields
of nerve growth factor-induced neurite outgrowth in PC-12 cells. Bioelectromagnetics 16:387-395.
Blackman CF, Blanchard JP, Benane SG, House DE (1994). Empirical test of an ion parametric resonance
model for magnetic field interactions with PC-12 cells. Bioelectromagnetics 15:239-260.
Blackman CF, Blanchard JP, Benane SG, House DE (1995). The ion parametric resonance model predicts
magnetic field parameters that affect nerve cells. FASEB 9:547-551.
Blackman CF, Blanchard JP, Benane SG, House DE (1999). Experimental determination of hydrogen
bandwidth for the ion parametric resonance model. Bioelectromagnetics 20(1):5- 12.
Blanchard JP, Blackman CF (1994). Clarification and application of an ion parametric resonance model
for magnetic interaction with biological systems. Biolectromagnetics 15:217-238.
Blank M, Soo L (1992). Threshold for inhibition of Na/K ATPase by ELF alternating currents. Biolectromagnetics 13:329-333.
Blank M, Soo L (1993a). The Na/K ATPase as a model for electromagnetic field effects on cells. Bioelectrochem Bioenerg 30:85-92.
Blank M, Soo L (1993b). The threshold for Na/K ATPase stimulation by electromagnetic fields. Bioelectroch Bioenerg 40:63-65.
Bobkova NV, Novikov VV, Medvinskaya NI, Fesenko EE (2005). Reduction in the 13-amyloid level in the brain under the action of weak combined fields in a model of Sporadic Alzheimer’s disease. Biophysics 540:52-57.
Brugemann H, editor. (1990). Bioresonance and multiresonance therapy (BRT). Brussels: Haug International Publishing.
Chou C-K, McDougall JA, Ahn C, Vora N (1997). Electrochemical treatment of mouse and rat fibrosarcomas with direct current. Bioelectromagnetics 18:14-24.
Cossarizza A, Monti D, Bersani F, Cadossi R, Sacchi Franceschi C (1989). Extremely low frequency pulsed electromagnetic fields increase cell proliferation in lymphocytes from young and aged subjects. Biochem Biophys Res Commun 160:692-698.
Cossarizza A, Monti D, Bersani F, Paganelli R, Montagnani G, Cadossi R, Cantini M, Franceschi C (1989). Extremely low frequency pulsed electromagnetic fields increase interleulcin-2 (IL-2) and IL-2 receptor expression in lymphocytes from old subjects. FEBS Lett 248:141-144.
Cossarizza A, Angioni S, Petraglia F, Genazzoni AR, Monti D, Capri M, Bersani F, et al. (1993). Exposure to low frequency pulsed electromagnetic fields increases interleukin-1 and interleukin-6 production by human peripheral blood mononuclear cells. Exp Cell Res 204:385-387.
Del Giudice E, Preparata G (1995). Coherent dynamics in water as a possible explanation of biological membranes formation. J Biol Phys 20:105-116.
Del Giudice E, Galimberti A, Gamberane L, Preparata G (1995). Electrodynamical coherence in water: a possible origin of the tetrahedral coordination. Mod Phys Lett B 9:953-961.
Del Giudice E, Fleischmann M, Preparata G, Talpo G (2002). On the `unreasonable’ effects of ELF magnetic fields upon a system of ions. Bioelectromagnetics 23:522-530.
Diebert MC, McLeod BR, Smith SD, Liboff AR (1994). Ion resonance electromagnetic field stimulation of fracture healing in rabbits with a fibular ostectomy. I Orthopedic Res 12:878-885.
Dun HP, Popp FA, Schommers W, editors. (2002). What is life? Scientific approach and philosophical positions. Singapore: World Scientific Publishing.
Eichwald CF, Kaiser F (1993). Model for receptor-controlled cytosolic calcium oscillations and for external influences on the signal pathway. Biophys J 65:2047-2058.
Eichwald CF, Walleczek J (1996a). Model for magnetic field effects on radical pair recombination in enzyme kinetics. Biophys J 71:623-631.
Eichwald CF, Walleczek J (l 996b). Activation-dependent and biphasic electromagnetic field effects: model based on cooperative enzyme kinetics in cellular signalling. Bioelectromagnetics 17:427-435.
Eichwald CF, Walleczek J (1997). Low-frequency-dependent effects of oscillating magnetic field effects of oscillating magnetic fields on radical pair recombination in enzyme kinetics. I Chem Phys 107: 4943 – 4950.
Eichwald CF, Walleczek J (1998). Magnetic field perturbations as a tool for controlling enzyme-regulated and oscillatory biochemical reactions. Biophys Chem 74:209-224.
Figiel GS, Epstein C, McDonald WM, Amazon-Leece J, Figiel L, Saldivia A, Glover S (1998). The use of rapid-rate transcranial magnetic stimulation (rTMS) in refractory depressed patients. I Neuropsychiatr Clin Neurosci 10:20-25.
Fitzsimmons RI, Ryaby IT, Mohan S, Magee FP, Baylink DG (1995). Combined magnetic fields increase insulin-like growth factor II in TE-85 human osteosarcoma bone cell cultures. Endocrinology 136: 3100-3106.
Foletti A, Lisi A, Ledda M, De Carlo F, Grimaldi S (2009). Cellular ELF signals as a possible tool in informative medicine. Electromagnetic Biol Med 28(1):71 – 79.
Frohlich H, editor. (1988). Biological coherence and response to external stimuli. Berlin/Heidelberg: Springer Verlag.
Frohlich H, Kremer F, editors. (1983). Coherent excitations in biological systems. Berlin/Heidelberg: Springer Verlag.
Fuchs EC, Woisetschlager J, Gatterer K, Maiser E, Pecnic R, Holler G, Eisenkolbl H (2007). The floating water bridge. J Phys D: Appl Phys 40:6112-6114.
Gaetani R, Ledda M, Barile L, Cimenti I, De Carlo F, Forte E, Ionta V, et al. (2009). Differentiation of human adult cardiac stem cells exposed to extremely low-frequency electromagnetic field. Cardiovasc Res 82: 411 – 420.
Galland P, Pazur A (2005). Magnetoreception in plants. Plant Res 118:371 -389.
George MS, Wassermann EM, Williams WA, Callahan A, Ketter TA, Basser P, Hallett M, Post RM (1995). Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport 2:1853-1856.
Giuliani L, Grimaldi S, Lisi A, D’Emilia E, Bobkova N, Zhadin M (2008). Action of combined magnetic fields on aqueous solution of glutamic acid: the further development of investigations. Biomagn Res Technol 6(1). doi:10.1186/1477-044X-6-1.
Giuliani L, D’Emilia E, Lisi A, Grimaldi S, Foletti A, Del Giudice E (2009). The floating water bridge under strong electric potential. Neural Netw World 19(4):393 -398.
Grimaldi S, Pasquali E, Barbanato L, Lisi A, Santoro N, Serafina A, Pozzi D (1997). Exposure to a 50 Hz electromagnetic field induces activation of the Epstein-Barr virus genome in latently infected human lymphoid cells. J Environ Pathol Toxicol Oncol 16:205-207.
Hoelzel R, Lamprecht J (1994). Electromagnetic fields around biological cells. Neural Network World 4: 327-337.
Hoelzel R, Lamprecht J (1995). Optimizing an electronic detection system for radiofrequency oscillations in biological systems. Neural Network World 5:763- 774.
Islamov BI, Funtikov VA, Bobrovski RV, Gotovskii YV (1999). Bioresonance therapy in rheumatoid arthritis and heat shock proteins. Bull Exp Biol Med 128(10:525-528.
Islamov BI, Balabanova RM, Funtikov VA, Gotovskii YV, Meizerov EE (2002). Effect of bioresonance therapy on antioxidant system in lymphocytes in patients with rheumatoid arthritis. Bull Exp Biol Med 134(3):248 – 250.
Jelinek F, Pokorny J, Saroch J, Trkal V, Hasek J, PaIan B (1999). Microelectronic sensors for measurement of
electromagnetic fields of living cells and experimental results. Biolectrochem Bioenerg 48:261-266.
Kaiser F (1996). External signals and internal oscillation dynamics: biophysical aspects and modelling approaches for interactions of weak electromagnetic fields at the cellular level. Bioelectrochem Bioeng 41:3-18.
Kirson ED, Dbaly V, Tovarys F, Vyrnazal J, Soustiel JF, Itzhaki A, Mordechovich D, et al. (2007). Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci USA 104:10152-10157.
Kirson ED, Giladi M, Gurvich Z, Itzhaki A, Mordechovich D, Schneiderman RS, Wasserman Y, et al. (2009). Alternating electric fields (TT fields) inhibit metastatic spread of solid tumors to the lungs. Clin Exp Metastasis 26:633 -640.
Lednev VV (1991). Possible mechanism for the influence of weak magnetic field on biological system. Bioelectromagnetics 12:71-75.
Liboff AR (1985). Geomagnetic cyclotron resonance in living cells. Biol Phys 13:99-102.
Liboff AR. The electromagnetic field as a biological variable. In: Frey AH, editor. On the nature of electromagnetic field interactions. Austin: R.G. Landis; 1994.
Liboff AR (1997). Electric-field ion cyclotron resonance. Biolectromagnetics 18()):85 – 87.
Liboff AR (2002). Comment on `Extremely low frequency magnetic fields can either increase or decrease
analgesia in the land snail depending on field and light conditions”. Bioelectromagnetics 23:406-407. Liboff AR (2004). Toward an electromagnetic paradigm for biology and medicine. J Altern Complement
Med 10(1):41 – 47.
Liboff AR. The on cyclotron resonance hypothesis. In: Greenebaum B, Barnes F, editors. Handbook of Bioelectromagnetism. 3rd ed. Boca Raton: CRC Press; 2006.
Liboff AR (2007). Local and holistic electromagnetic therapies. Electromagn Biol Med 26(4):315 – 325. Liboff AR (2010). A role for the geomagnetic field in cell regulation. Electromagn Biol Med 29:105-112. Liboff AR (2012). Electromagnetic vaccination. Med Hypotheses 79(3):331 – 333.
Liboff AR, Jenrow KA (2002). Physical mechanisms in neuroelectromagnetic therapies. Neuro Rehabilitation 17:9-22.
Liboff AR, Rozek RI, Sherman ML, McLeod BR, Smith SD ()987). Ca2-45 cyclotron resonance in human lymphocytes. Electromag Biol Med 6:13 – 22.
Liboff AR, Smith S, Mc Leod B ()995). Comments on “Clarification and application of an Ion Parametric Resonance model for magnetic field interactions with biological systems,” by Blanchard and Blackman. Biolectromagnetics 16:272-273.
Liburdy RP (1992). Calcium signalling in lymphocytes and ELF fields: evidence for an electric field metric and a site of interaction involving calcium ion channels. FEBS Lett 301:53-59.
Lisi A, Ledda M, Rosola E, Pozzi D, D’Emilia E, Giuliani L, Foletti A, et al. (2006). Extremely low frequency electromagnetic field exposure promotes differentiation of pituitary corticotrope-derived AtT20 D16V cells. Bioelectromagnetics 27:641-651.
Lisi A, Foletti A, Ledda M, Rosola E, Giuliani L, D’Emilia E, Grimaldi S (2006). Extremely low frequency 7 Hz 1001.1T electromagnetic radiation promotes differentiation in the human epithelial cell line HaCaT. Electromagn Biol Med 25(4):269 – 280.
Lisi A, Rieti S, Criceti A, Flori A, Generosi R, Luce M, Perfetti P, et al. (2006). ELF non-ionizing radiation changes the distribution of the inner chemical functional groups in human epithelial cell (HaCaT) culture. Electromagn Biol Med 25(4):281 – 289.
Lisi A, Ledda M, De Carlo F, Pozzi D, Messina E, Gaetani R, Cimenti I, et al. (2008). Ion cyclotron resonance as a tool in regenerative medicine. Electromagn Biol Med 27:127-133.
Lis/A, Ledda M, De Carlo F, Foletti A, Giuliani L, D’Emilia E, Grimaldi S (2008). Ion cyclotron resonance (ICR) transfers information to living systems: effects on human epithelial cell differentiation. Electromagn Biol Med 27(3):230- 240.
Lyle DB, Wang X, Ayotte RD, et al. (1991). Calcium uptake by leukemic and normal T-lymphocytes exposed to low frequency magnetic fields. Bioelectromagnetics 12:145-155.
Mae-Wan Ho (1998). The rainbow and the worm. The physics of the organisms. Singapore: World Scientific Publishing.
Markin VS, Tsong TY (1991a). Frequency and concentration windows for the electric activation of a membrane active transport system. Biophys 59(6):1308 – 1316.
Markin VS, Tsong TY (1991b). Reversible mechanosensitive ion pumping as a part of mechanoelectric transduction. Biophys J 59(6):1317- 1324.
Markin VS, Tsong TY (1991c). Electroconformational coupling for ion transport in an oscillating electric field: rectification versus active pumping. Bioelectrochem Bioenerg 26:251 -276.
Matteucci C (1834). Memoire sur la l’electrite animal. Annales de Chimie et de Physique 56:449-443. McLeod BR, Liboff AR (1986). Dynamic characteristics of membrane ions in multifield configurations of low-frequency electromagnetic radiation. Bioelectromagnetics 7:177-189.
Novikov VV, Novikov GV, Fesenko EE (2009). Effect of weak combined static and extremely low-frequency alternating magnetic fields on tumor growth in mice inoculated with the Ehrlich ascites carcinoma. Bioelectromagnetics 30:343-351.
Pazur A (2004). Characterisation of weak magnetic field effects in an aqueous glutamic acid solution by nonlinear dielectric spectroscopy and voltammetry. Biomagn Res Technol 2(8) doi:10.1186/1477-044X-2-8
Pelling AE, Sehati S, GraIla EB, Valentine JS, Gimzewslci JK (2004). Local nanomechanical motion of the cell wall of Saccharomyces cerevisiae. Science 305(5687):1147- 1150.
Pelling AE, Sehati S, GraIla EB, Gimzewski JK (2005). Time dependence of the frequency and amplitude of the local nanomechanical motion of yeast. Nanomedicine 1(2):178-183.
Pelling AE, Veraitch FS, Pui-Ke Chu C, Nicholls BM, Hemsley AL, Mason C, Horton MA (2007). Mapping correlated membrane pulsations and fluctuations in human cells. J Mol Recognit 20(6): 467-475.
Pohl HA (1980). Oscillating fields about growing cells. Int Quant Chem Quant Biol Symp 7:411 431. Pohl HA (1981). Natural electrical RF oscillation from cells. J Bioenerg Biomembr 13:149-169. Pokorny) (1999). Conditions for coherent vibrations in the cytoskeleton. Bioelectrochem Bioenerg 48:
267-271.
Pokorny J (2001). Endogenous electromagnetic forces in living cells: implications for transfer of reaction components. Electro Magnetobiol 20(1):59- 73.
Pokorny J, Wu TM (1998). Biophysical aspects of coherence and biological order. Berlin/Heidelberg/ New York: Springer Verlag.
Pokorny J, Jelinek F, Trkal V (1998). Electric field around microtubules. Bioelectrochem Bioenerg 45: 239-245.
Popp FA, Beloussov L, editors. (2003). Integrative biophysics. Biophotonics. Dordrecht/Boston/London: Kluwer Academic Publishers.
Preparata G (1995). QED coherence in matter. Singapore: World Scientific Publishing.
Presman A (1970). Electromagnetic fields and life. New York: Plenum Press.
Raggi F, G, Rufini S, Gizzi S, Ercolani E, Rossi B (2008). ELF magnetic therapy and oxidative balance.
Electromagn Biol Med 27:325-339.
Rapp PE (1979). An atlas of cellular oscillators. J Exp Biol 81:281-306.
Rapp PE (1980). The origin and function of cellular oscillations. Cell Biol Int Rep 4(2):227- 229.
Regling C, Brueckner C, Kimura JH, Liboff AR (2002). Evidence for ICR magnetic field effects on cartilage
and bone development in embryonic chick bone explants. 48th annual meeting, Orthopedic Research
Society, Dallas.
Ross SM (1990), Combined DC and ELF magnetic fields can alter cell proliferation. Bioelectromagnetics 11:27-36.
Rossi EW, Corsetti MT, Sukkar S, Poggi C (2007). Extremely low frequency electromagnetic fields prevent chemotherapy induced myelotwdcity. Electromagn Biol Med 26:277-281.
Rozek RJ, Sherman ML, Liboff AR, McLeod BR, Smith S, D (1987). Nifedipine is an antagonist to cyclotron resonance enhancement of 45Ca incorporation in human lymphocytes. Cell Calcium 8: 413 – 427.
Rubik B (1995). Energy medicine and the unifying concept of information. Altern Ther Health Med 1(1): 34-39.
Sahu S, Hirata K, Fujuita D, Ghosh S, Bandyopadhyay A (2012). Radio-frequency induced ultrafast assembly of microtubules and their length-independent electronic properties. Nat Matter (in press).
Santoro N, Lisi A, Pozzi D, Pasquali E, Serafino A, Grimaldi S (1997). Effect of extremely low frequency (ELF) magnetic field exposure on morphological and biophysical properties of human lymphoid cell line (Raji). Biochim Biaphys Acta 1357:281-290.
Sarimov R, Markova E, Johansson, Jenssen D, Belyaev I (2005). Exposure to ELF magnetic field tuned to Zinc inhibits growth of cancer cells. Bioelectromagnetics 26:631-638.
Sarimov R, Alipov ED, Belyaev IY (2011). Fifty hertz magnetic fields individually affect chromatin conformation in human lymphocytes: dependence on amplitude, temperature, and initial chromatin state. Bioelectromagnetics 32:570-579.
Schrodinger E (1944). What is life? The physical aspect of the living cell. Cambridge: Cambridge University Press.
Smith SD, McLeod BR, Liboff AR, Cooksey K (1987). Calcium cyclotron resonance and diatom motility. Bioelectromagnetics 8:215-227.
Smith SD, Liboff AR, McLeod BR (1991). Effects of resonant magnetic fields on chick femoral development in vitro. Electromag Biol Med 10:81 -89.
Smith SD, Liboff AR, McLeod BR. Effects of resonance tuned magnetic field on N-18 neuroblastoma cells. In: Allen M, Cleary S, Sowers AE, Shillady DD, editors. Charge and field effects in biosystems-3. Boston: Birkhauser; 1992.
Smith SD, McLeod BR, Liboff AR (1993). Effects of CR-tuned 60 Hz magnetic fields on sprouting and early growth of Raphanus sativus. Bioelectrochem Bioenerg 32:67-76.
Smith SD, McLeod BR, Liboff AR (1995). Testing the ion cyclotron resonance theory of electromagnetic
field interaction with odd and even harmonic tuning for cations. Bioelectrochem Bioenerg 38:161-167. Stuart CIJ (1985a). Physical models of biological information and adaptation. J Theor Biol 113:441 -454. Stuart CU (1985b). Bio-informational equivalence. 1 Theor Biol 113:611-636.
Thomas JR, Schrot J, Liboff AR (1986). Low-intensity magnetic fields alter operant behaviour in rats. Bioelectromagnetics 7:349-357.
Tsong TY (1990). Electrical modulation of membrane proteins: enforced conformational oscillations and
biological energy and signal transduction. Annu Rev Biophys Biophys Chem 19:83-106.
Tsong TY (1992). Molecular recognition and processing of periodic signals in cells: study of activation of
membrane ATPases by alternating electric fields. Biochim Biophys Acta 1113(1):53-70.
Tsong TY, Liu DS, Chauvin F, Astumian RD (1989). Resonance electroconformational coupling: a proposed
mechanism for energy and signal transductions by membrane proteins. Biosci Rep 9(1):13 -26.
Vincze G, Szasz N, Szasz A (2005). On the thermal noise limit of cellular membranes. Bioelectromagnetics
26:28-35.
Vincze G, Szasz A, Liboff AR (2008). New theoretical treatment of ion resonance phenomena. Bioelectromagnetics 29(5):380 -386.
Volodyaev I (2005). Bridging the gap between physics and biology. Riv Biol 98(2):237 – 264.
Walleczek (1992). Electromagnetic field interactions with cells of the immune system: the role of calcium signaling. FASEB J 6:3177-3185.
Weaver JC, Astumian RD (1990). The response of living cells to very weak magnetic fields: the thermal noise limit. Science 247:459-462.
Yost MG, Liburdy RP (1992). Time-varying and static magnetic fields act in combination to alter calcium signal transduction in the lymphocyte. FEBS Lett 296:117-122.
Zewail AhmedH, editor. (2008). Physical biology: from atoms to medicine. London: Imperial College Press. Zhadin MN (1996). Effect of magnetic fields on the motion of an ion in a macromolecule: theoretical analysis. Biophysics 41:843-860.
Zhadin MN (1998). Combined action of static and alternating magnetic fields on ion motion in a macromolecule: theoretical aspects. Bioelectromagnetics 19:279-292.
Zhadin MN (2001). Review of Russian literature on biological action of DC and low-frequency AC magnetic fields. Bioelectromagnetics 22(1):27 -45.
Zhadin MN, Barnes F (2005). Frequency and amplitude windows at combined action of DC and low frequency AC magnetic Fields on ion thermal motion in a macromolecule: theoretical analysis. Bioelectromagnetics 26:323-330.
Zhadin MN, Fesenko EE (1990). Ion cyclotron resonance in biomolecules. Biomed Sci 1(3):245- 250. Zhadin MN, Giuliani L (2006). Some problems in modern bioelectromagnetics. Electromagn Biol Med 25: 227 – 243.
Zhadin MN, Novikov, VV, Barnes FS, Pergola NF (1998). Combined action of static and alternating magnetic fields on ionic current in aqueous glutamic acid solution. Bioelectromagnetics 19:41-45.
Zhadin MN, Deryugina ON, Pisachenko TM (1999). Influence of combined DC and AC magnetic fields on rat behavior. Bioelectromagnetics 20:378-386.

